
What are mixed anhydrides?
Answer
520.5k+ views
Hint: In the presence of Oxygen, almost all the nonmetals form a product called metal oxide. When non-metal oxides react with water they produce acid as the product and when non-metal oxides react with base they produce salt with water.
Complete answer:
Mixed anhydrides, as the name suggests, are the mixture of more than two anhydrides and anhydrides, we can say that they are the compounds which we obtain by the removal of the water molecules from that particular compound.
For example: acetic acid anhydride- now, we know what is the structure of acetic acid:
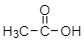
Now, let’s see the structure for anhydride of acetic acid:
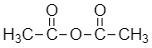
Anhydride of acetic acid is formed when two molecules of acetic acid lose their water molecule.
Therefore, we can say that mixed anhydrides are those compounds which react with molecules of water in order to form a mixture containing two acidic anhydrides. They are the derivatives of acid with a general structural formula:
$RC( = O)O(O = C){R^{}}$’
For example: When nitrogen oxide reacts with water molecule it will from two acids:
$2N{O_2} + {H_2}O \to HN{O_2} + HN{O_3}$
Here, two acids which are formed are known as nitrous acid $(HN{O_2})$ and nitric acid $(HN{O_3})$.
Let’s see some more examples for mixed anhydrides:
When chlorine dioxide reacts with water. Chlorine dioxide is a compound having formula $Cl{O_2}$and it exists in the form of a gas having yellowish-green colour and in liquid state, it exists with reddish-brown colour and in crystal state, it exists with bright-orange colour.
$2Cl{O_2} + {H_2}O \to HCl{O_2} + HCl{O_3}$
It forms two acids called chlorous acid $(HCl{O_2})$ and chloric acid $(HCl{O_3})$.
Hence, we can say that mixed anhydrides are the chemical compounds which react with water molecules to produce two or more acids.
Note:
Every formed mixed anhydride has their different properties. They are further classified into symmetrical and unsymmetrical anhydrides on the basis of their geometry.
Examples of symmetrical anhydride are propionic anhydride and acetic anhydride,
Examples of unsymmetrical anhydride are acetic propionic anhydride and acetic butyric anhydride.
Complete answer:
Mixed anhydrides, as the name suggests, are the mixture of more than two anhydrides and anhydrides, we can say that they are the compounds which we obtain by the removal of the water molecules from that particular compound.
For example: acetic acid anhydride- now, we know what is the structure of acetic acid:
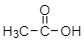
Now, let’s see the structure for anhydride of acetic acid:
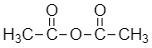
Anhydride of acetic acid is formed when two molecules of acetic acid lose their water molecule.
Therefore, we can say that mixed anhydrides are those compounds which react with molecules of water in order to form a mixture containing two acidic anhydrides. They are the derivatives of acid with a general structural formula:
$RC( = O)O(O = C){R^{}}$’
For example: When nitrogen oxide reacts with water molecule it will from two acids:
$2N{O_2} + {H_2}O \to HN{O_2} + HN{O_3}$
Here, two acids which are formed are known as nitrous acid $(HN{O_2})$ and nitric acid $(HN{O_3})$.
Let’s see some more examples for mixed anhydrides:
When chlorine dioxide reacts with water. Chlorine dioxide is a compound having formula $Cl{O_2}$and it exists in the form of a gas having yellowish-green colour and in liquid state, it exists with reddish-brown colour and in crystal state, it exists with bright-orange colour.
$2Cl{O_2} + {H_2}O \to HCl{O_2} + HCl{O_3}$
It forms two acids called chlorous acid $(HCl{O_2})$ and chloric acid $(HCl{O_3})$.
Hence, we can say that mixed anhydrides are the chemical compounds which react with water molecules to produce two or more acids.
Note:
Every formed mixed anhydride has their different properties. They are further classified into symmetrical and unsymmetrical anhydrides on the basis of their geometry.
Examples of symmetrical anhydride are propionic anhydride and acetic anhydride,
Examples of unsymmetrical anhydride are acetic propionic anhydride and acetic butyric anhydride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




