
what is meant by chiral centre?
Answer
573.6k+ views
Hint: It is the fundamental requirement for an optically active molecule to show optical isomerism. The spatial arrangement of atoms decides the chiral centre and the groups of atoms present in it.
Complete answer:
We know that an atom attaches to other atoms to form a molecule and compounds. Chiral centre is the atom which is attached to four different groups which gives a mirror image which is non-superimposable.
-Now we refer to the chiral centre as chirality. Chirality is mainly observed due to the three dimensional spatial arrangements of different groups of atoms.
-Example of a chiral centre
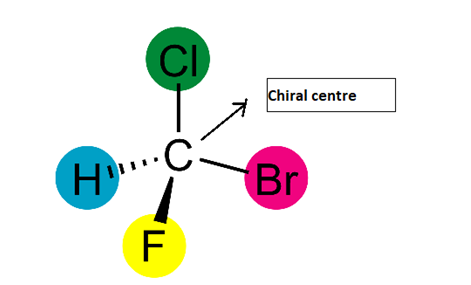
-A carbon atom surrounded by halogens and hydrogen, here the carbon is a chiral carbon and produces non-superimposable mirror image. As this possesses a chiral centre it will be optically active i.e. it rotates plane polarized light.
-Chiral substances are asymmetric in nature and the mirror images are non –superimposable like the mirror images of our legs, hands. Chiral centre is also called a stereogenic centre.
-Achiral objects are symmetric in nature and form superimposable mirror images like cone shaped objects.
Additional information:
Stereoisomerism or spatial isomerism is the isomerism in which the molecules have the same formulae but they differ in the spatial arrangement. They are mainly classified as enantiomers and diastereomers.
-Molecules that have mirror images but are non-superimposable are enantiomers
-Molecules that are not both mirror images and non-superimposable are called diastereomers.
Note: The determination of the chiral centre should be given great concern as the C must attach to four different atoms to show chirality. Double bonded or triple bonded carbon can’t be chiral since they can make four bonds. Likewise carbon straight chains also can’t make a chiral centre as there will be at least one repeating atom say H.
Complete answer:
We know that an atom attaches to other atoms to form a molecule and compounds. Chiral centre is the atom which is attached to four different groups which gives a mirror image which is non-superimposable.
-Now we refer to the chiral centre as chirality. Chirality is mainly observed due to the three dimensional spatial arrangements of different groups of atoms.
-Example of a chiral centre

-A carbon atom surrounded by halogens and hydrogen, here the carbon is a chiral carbon and produces non-superimposable mirror image. As this possesses a chiral centre it will be optically active i.e. it rotates plane polarized light.
-Chiral substances are asymmetric in nature and the mirror images are non –superimposable like the mirror images of our legs, hands. Chiral centre is also called a stereogenic centre.
-Achiral objects are symmetric in nature and form superimposable mirror images like cone shaped objects.
Additional information:
Stereoisomerism or spatial isomerism is the isomerism in which the molecules have the same formulae but they differ in the spatial arrangement. They are mainly classified as enantiomers and diastereomers.
-Molecules that have mirror images but are non-superimposable are enantiomers
-Molecules that are not both mirror images and non-superimposable are called diastereomers.
Note: The determination of the chiral centre should be given great concern as the C must attach to four different atoms to show chirality. Double bonded or triple bonded carbon can’t be chiral since they can make four bonds. Likewise carbon straight chains also can’t make a chiral centre as there will be at least one repeating atom say H.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




