
Which of the following fischer projection(s) represent L-glucose?
(A)
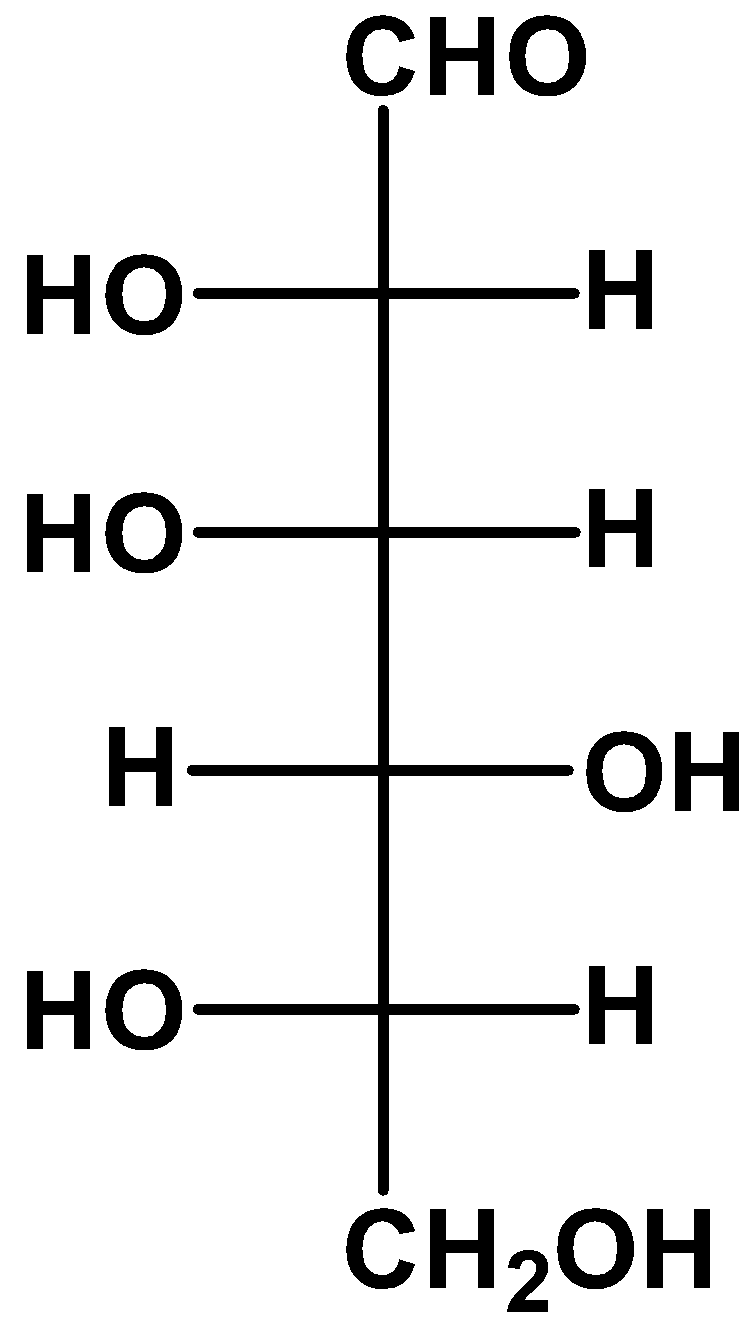
(B)
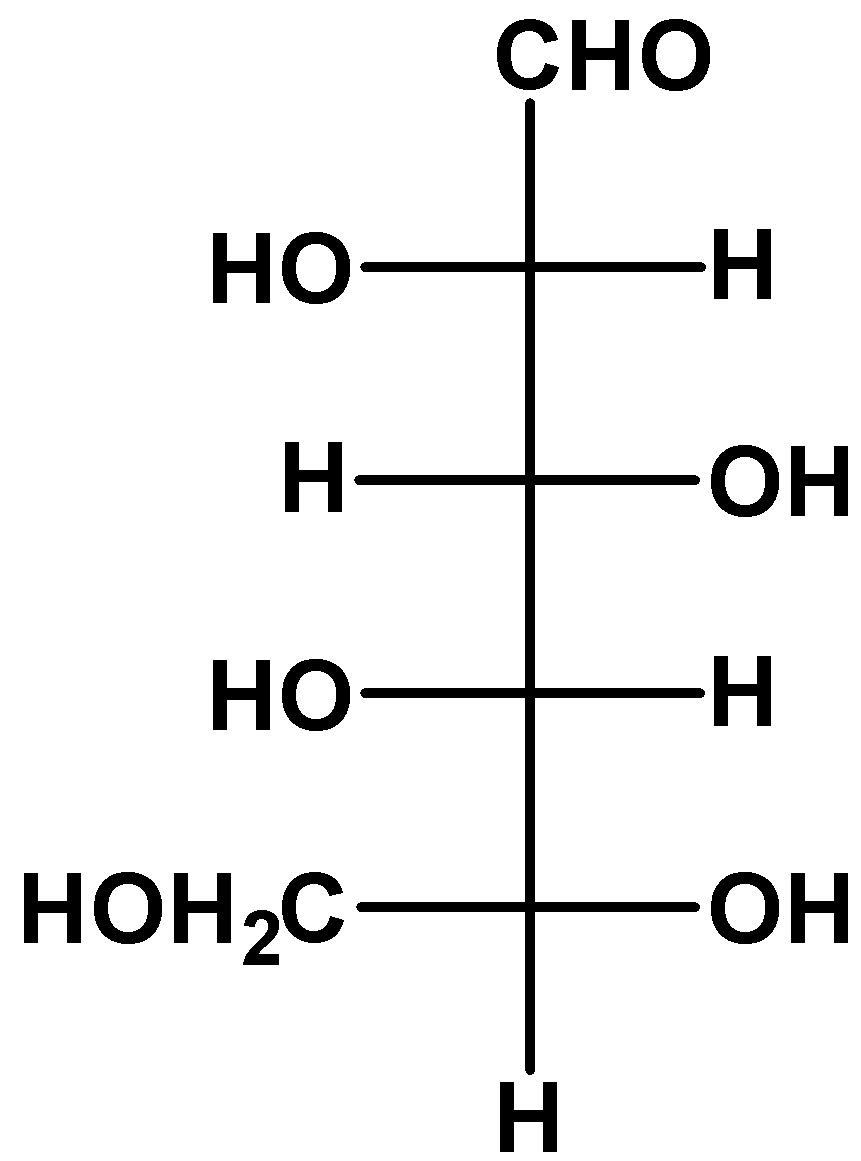
(C)
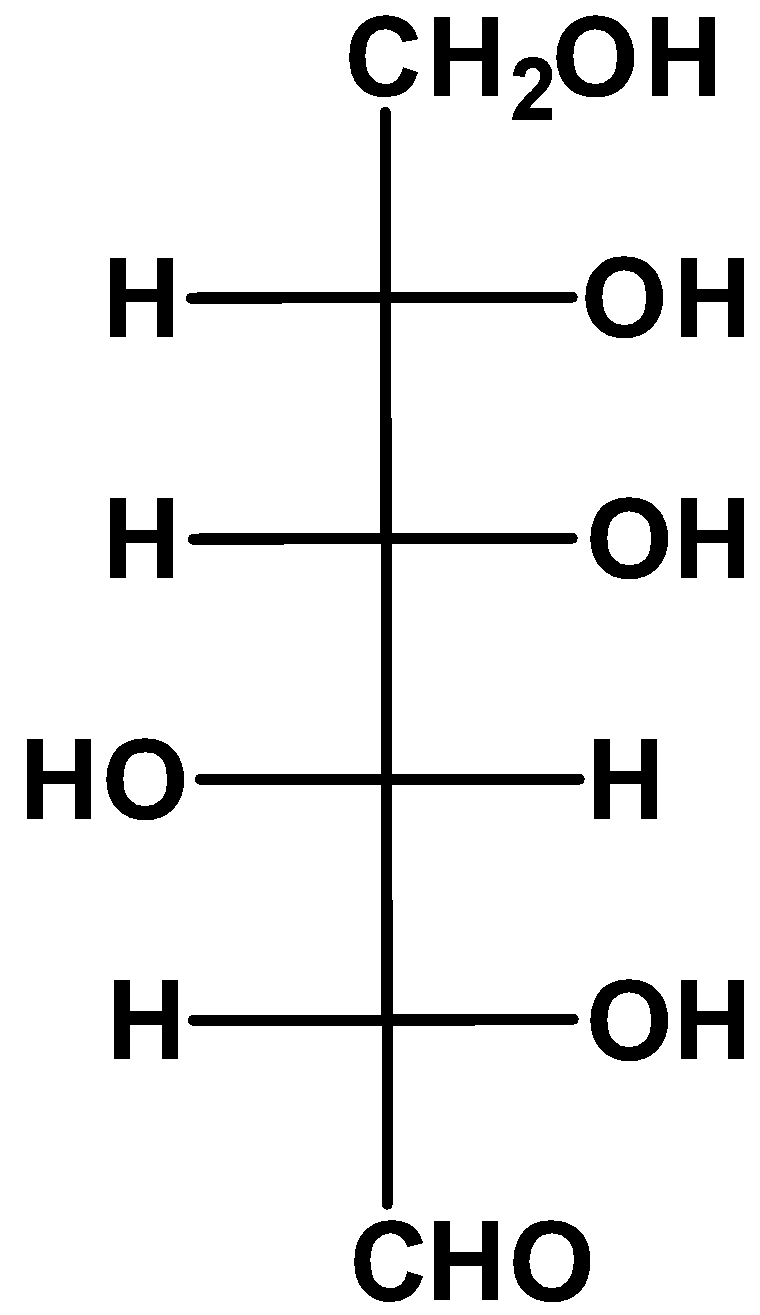
(D)
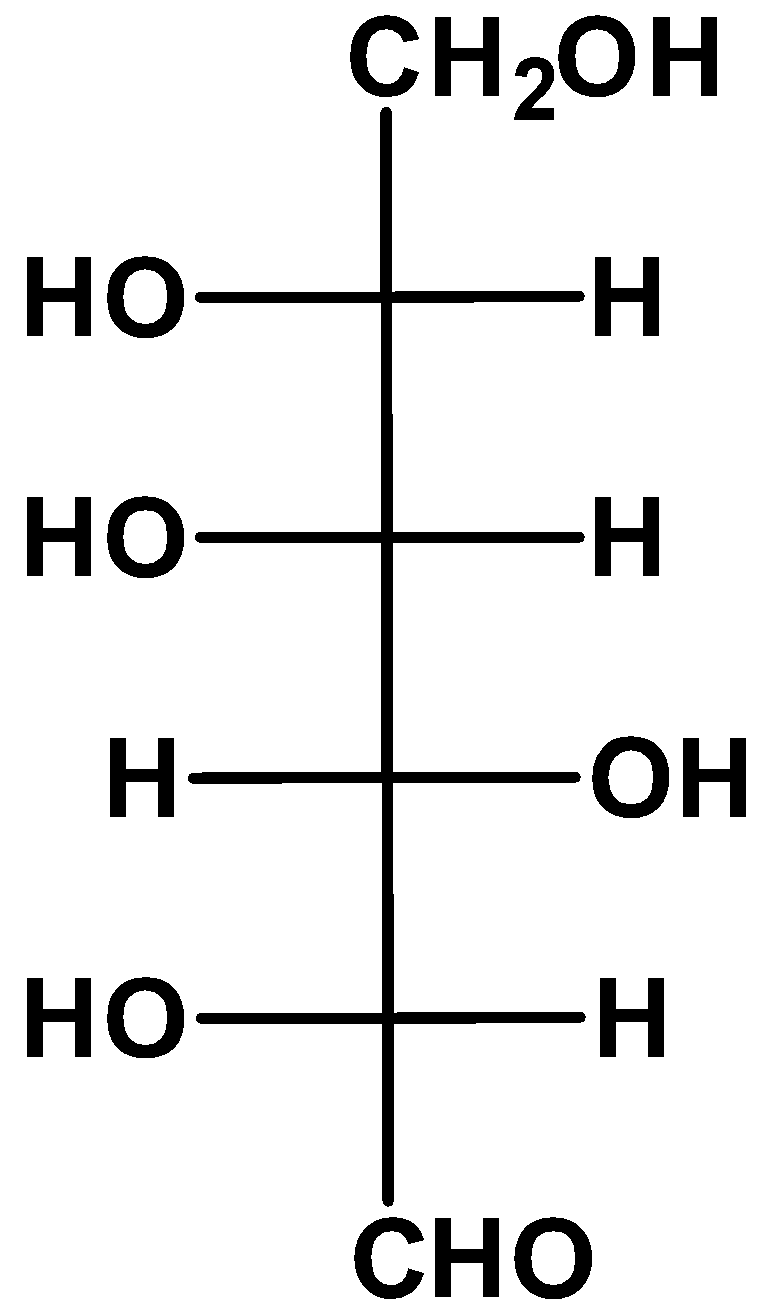




Answer
582.9k+ views
Hint: The trick is to know the position of the hydroxyl group on the second-last carbon atom; if it happens to be on the left hand side then we got our answer. If the structure is less obvious or not given in its conventional form you have to arrange the fischer projection in a way that does not change its overall configuration and stereochemical properties and then look for the position of hydroxyl atom.
Complete step-by-step answer:
The L and D configurations do not relate to the stereochemical properties of a molecule. What we mean by that is, they are not responsible for the determination of the direction in which the concerned molecules will rotate an incoming plane polarised light. These absolute configurations are named so in relation to the structure of glyceraldehyde. We have shown it as below-

As you can see above, the position of the hydroxyl group is taken into account. The configuration which is Laevorotatory or L is the one where hydroxyl group is on the left hand side, whereas the Dextrorotatory or D configuration is the one where we have hydroxyl group on the right-hand side. For all the structures used in biochemistry, the groups on the second-last carbon are compared to that of the glyceraldehyde molecule for determining their absolute configurations.
Here we have the glucose molecule in its fischer projection. From the rules we have known up until now, it is clear that option (A) is L-glucose.
But there are more and we can find them if we know some tricks to rotate the fischer projection without changing the overall stereochemistry of the molecule. Let us dive right into that.
- we take a look at option (B)
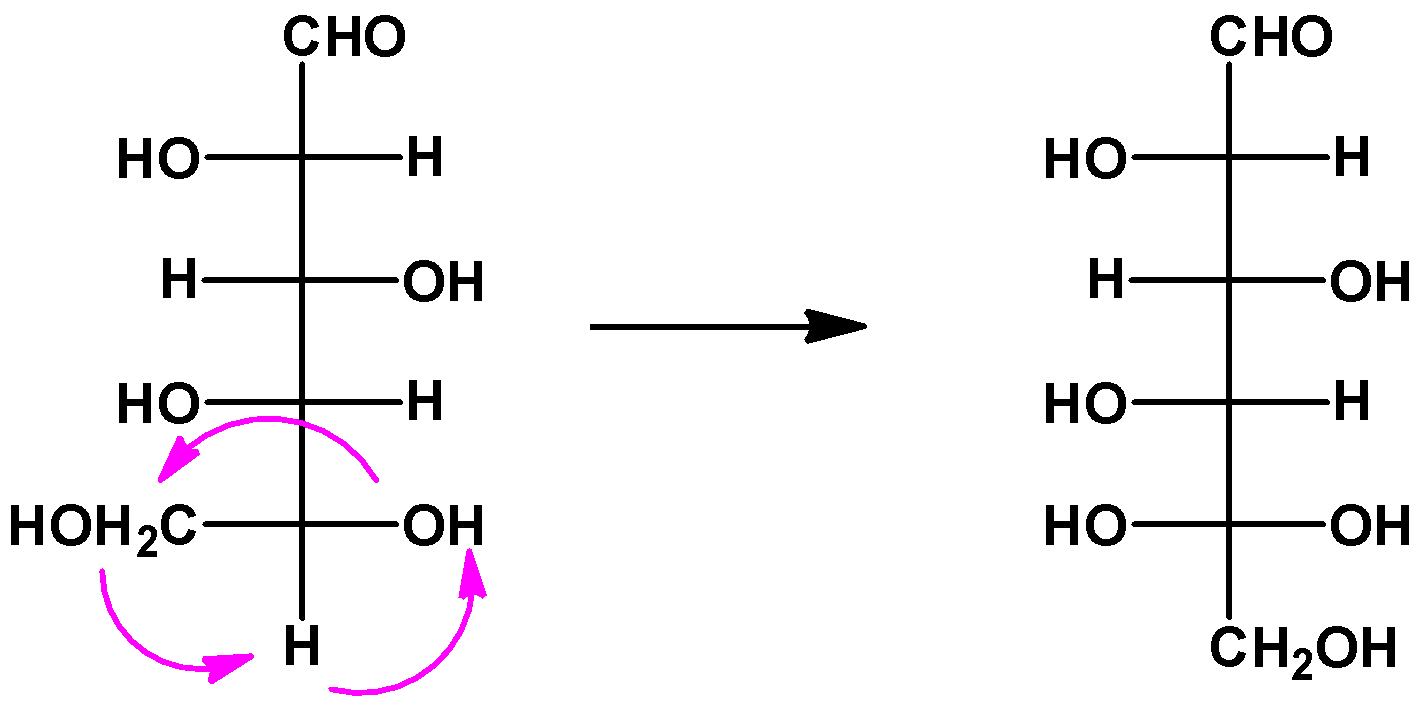
If we keep the position of one of the substituents fixed and rotate the other ones, then the resulting molecule will retain its stereochemistry as the original one. Here, we kept the position of the fourth carbon fixed and rotated the other substituents and we got an L-glucose as a result.
- we take a look at option (C)

The molecule is upside down as you can see. If we rotate the molecule through 180 degrees on the plane of paper, then we are not changing its stereochemical properties. When we do that, the resulting molecule is an L-glucose.
So, our answers are option (A), (B) and (C).
Note: Students should always keep in mind that all of the above rotations are done on the plane of paper. You cannot rotate molecules if the rotation involves taking the molecule out of the plane of paper. If we do so, we will produce a molecule which would not have the same stereochemistry.
Complete step-by-step answer:
The L and D configurations do not relate to the stereochemical properties of a molecule. What we mean by that is, they are not responsible for the determination of the direction in which the concerned molecules will rotate an incoming plane polarised light. These absolute configurations are named so in relation to the structure of glyceraldehyde. We have shown it as below-

As you can see above, the position of the hydroxyl group is taken into account. The configuration which is Laevorotatory or L is the one where hydroxyl group is on the left hand side, whereas the Dextrorotatory or D configuration is the one where we have hydroxyl group on the right-hand side. For all the structures used in biochemistry, the groups on the second-last carbon are compared to that of the glyceraldehyde molecule for determining their absolute configurations.
Here we have the glucose molecule in its fischer projection. From the rules we have known up until now, it is clear that option (A) is L-glucose.
But there are more and we can find them if we know some tricks to rotate the fischer projection without changing the overall stereochemistry of the molecule. Let us dive right into that.
- we take a look at option (B)

If we keep the position of one of the substituents fixed and rotate the other ones, then the resulting molecule will retain its stereochemistry as the original one. Here, we kept the position of the fourth carbon fixed and rotated the other substituents and we got an L-glucose as a result.
- we take a look at option (C)

The molecule is upside down as you can see. If we rotate the molecule through 180 degrees on the plane of paper, then we are not changing its stereochemical properties. When we do that, the resulting molecule is an L-glucose.
So, our answers are option (A), (B) and (C).
Note: Students should always keep in mind that all of the above rotations are done on the plane of paper. You cannot rotate molecules if the rotation involves taking the molecule out of the plane of paper. If we do so, we will produce a molecule which would not have the same stereochemistry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




