
Which one of the following structures represents nylon-6,6 polymer?
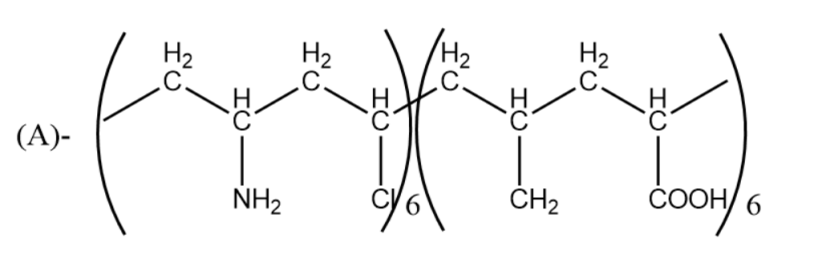
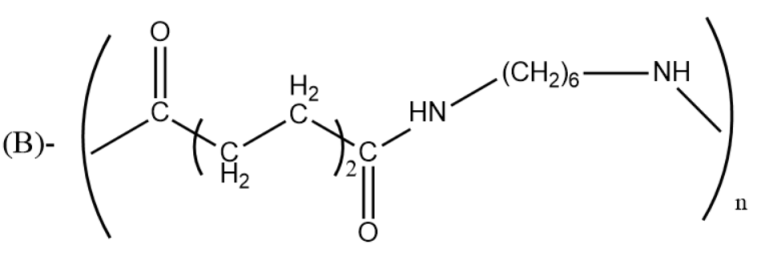
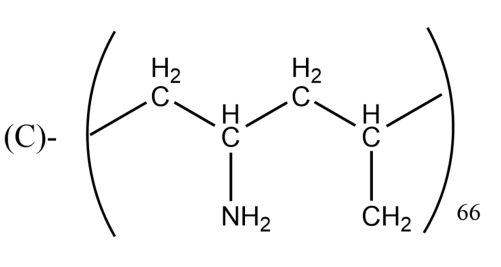
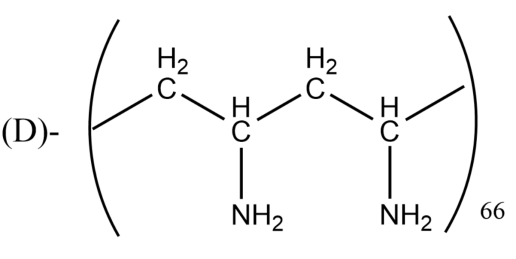




Answer
592.5k+ views
Hint: Nylon 6,6 is a type of polyamide or nylon which is made of two monomers each containing 6 carbon atoms. It is frequently used in the fibers of the textiles industry when high mechanical strength, rigidity, and good stability under heat and/or chemical resistance is required.
Complete answer:
-Polyamide is a synthetic polymer made by one molecule of amino acid and a carboxylic acid group of other molecules linked together.
-Polyamides can be naturally occurring or artificially synthesized. Examples of naturally occurring polyamides include proteins, such as wool and silk while the artificially synthesized polyamides can be made through step-growth polymerization yielding materials like nylons, aramids, etc.
-Polyamide derived its nomenclature from the number of carbon atoms contained in the diamine followed by the number of carbon atoms contained in the diacid.
-Polyamide 66, or nylon 6,6 is synthesized by polycondensation of hexamethylenediamine (six carbon atoms) and adipic acid (six carbon atoms).
The synthesis of Nylon 6,6 starts from the two comonomers which are reacted to form a salt. The purified salt having stoichiometric amounts of acids and amine is then polymerized at high temperatures and then is subjected to vacuum for dehydration to a high molecular weight polymer.
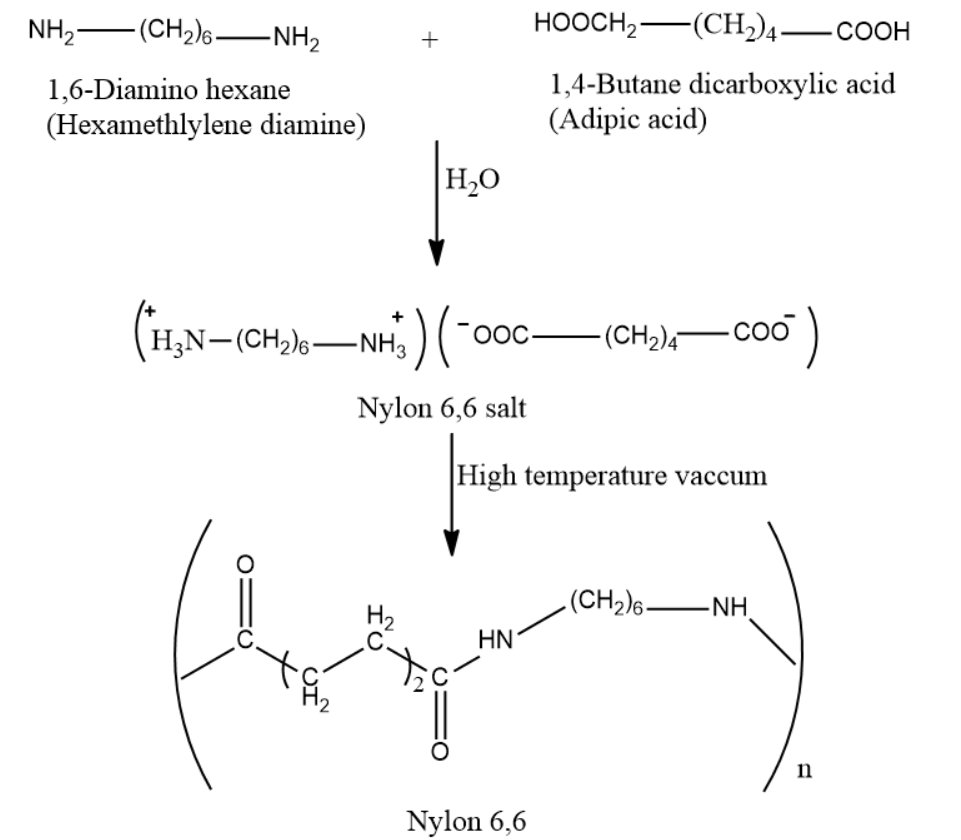
So, the correct answer is “Option B”.
Note: You may think that Nylon 6 and Nylon 6,6 are both the same, but it is not so. Nylon 6 is made up of one monomer which has 6 carbon hence named Nylon 6, whereas Nylon 6,6 is made from 2 monomers (comonomers) with each having six carbons hence named Nylon 6,6.
Complete answer:
-Polyamide is a synthetic polymer made by one molecule of amino acid and a carboxylic acid group of other molecules linked together.
-Polyamides can be naturally occurring or artificially synthesized. Examples of naturally occurring polyamides include proteins, such as wool and silk while the artificially synthesized polyamides can be made through step-growth polymerization yielding materials like nylons, aramids, etc.
-Polyamide derived its nomenclature from the number of carbon atoms contained in the diamine followed by the number of carbon atoms contained in the diacid.
-Polyamide 66, or nylon 6,6 is synthesized by polycondensation of hexamethylenediamine (six carbon atoms) and adipic acid (six carbon atoms).
The synthesis of Nylon 6,6 starts from the two comonomers which are reacted to form a salt. The purified salt having stoichiometric amounts of acids and amine is then polymerized at high temperatures and then is subjected to vacuum for dehydration to a high molecular weight polymer.

So, the correct answer is “Option B”.
Note: You may think that Nylon 6 and Nylon 6,6 are both the same, but it is not so. Nylon 6 is made up of one monomer which has 6 carbon hence named Nylon 6, whereas Nylon 6,6 is made from 2 monomers (comonomers) with each having six carbons hence named Nylon 6,6.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




