
With increase in temperature of a semiconductor its conductivity________________.
Answer
591.6k+ views
Hint: Semiconductor contains two words- semi means imperfect/moderate and conductor means the presence of a free electron. A semiconductor is a type of material in solid-state physics. With an increase in temperature, more electrons get the energy to jump from conduction to valence band and conductivity depends on the number of free/energetic electrons.
Complete step by step answer:
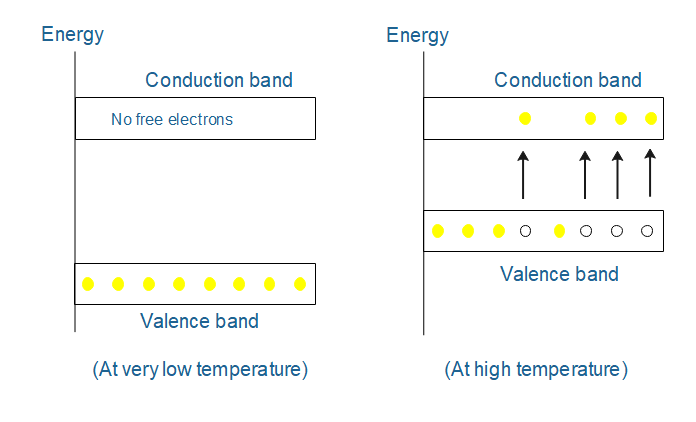
Basically, materials in solid-state physics are classified into three types based on conductivity: the insulator, semiconductor, and conductor. But before going into differences, we need to understand the concept of the forbidden energy gap. The forbidden energy gap plays a vital role in defining the electrical conductivity of the material.
Forbidden energy gap: energy gap between the valence band and conduction band is called the forbidden energy gap.
Now questions come: what is valence and conduction band. So valence band is a band where electrons having low energy are presently called valence electrons. Basically, it is an outermost electron of an atom and the conduction band is a band where electrons having high energy are presently called free electrons. An insulator, the forbidden energy gap between the valence band and conduction band is greater such that electrons from the valence band cannot jump into the conduction band. In a conductor, the forbidden energy gap between the valence band and conduction band is zero meaning the valence and conduction band is overlapped.
While in semiconductor, the forbidden energy gap between the valence band and conduction band is less. Therefore conductivity of semiconductors lies between the insulator and conductor. Examples of semiconductors are Silicon, germanium, arsenic, etc. The forbidden gap in a semiconductor is of the order of kT. At very low temperatures, the valence band is completely occupied by the valence electron and the conduction band is completely empty because no sufficient energy is present in the valence electron to jump into the conduction band. Therefore the forbidden energy gap remains the same as in insulators. Hence conduction won’t be possible and therefore the semiconductor acts as an insulator.
When the temperature increases, the top level of valence electron from valence electron gains some thermal energy i.e. kT greater than forbidden energy Eg. Hence a valence electron becomes a free electron and jumps into the conduction band which is responsible for the decrease in the forbidden energy gap and leads to conduction.
After the ejection of an electron from the valence band, a deficiency of electron is generated. It is known as a hole and acts as a positive charge. Thus the transfer of an electron from the valence to conduction creates an equal number of holes in the valence band and an equal number of electrons in the conduction band. These holes also cause decrees in the gap and hence conduction. Therefore as the temperature gets increases, more and more free electrons would be generated and this will cause, decrease in the forbidden energy gap between the valence band and conduction band.
Therefore, with an increase in temperature of a semiconductor its conductivity increases.
Note: Semiconductors have a negative temperature coefficient i.e. resistance of semiconductor decreases with an increase in temperature. The addition of suitable metallic impurity causes a change in conductivity. In the case of extrinsic semiconductors, the number of majority carriers is nearly constant, but mobility decreases.
Complete step by step answer:

Basically, materials in solid-state physics are classified into three types based on conductivity: the insulator, semiconductor, and conductor. But before going into differences, we need to understand the concept of the forbidden energy gap. The forbidden energy gap plays a vital role in defining the electrical conductivity of the material.
Forbidden energy gap: energy gap between the valence band and conduction band is called the forbidden energy gap.
Now questions come: what is valence and conduction band. So valence band is a band where electrons having low energy are presently called valence electrons. Basically, it is an outermost electron of an atom and the conduction band is a band where electrons having high energy are presently called free electrons. An insulator, the forbidden energy gap between the valence band and conduction band is greater such that electrons from the valence band cannot jump into the conduction band. In a conductor, the forbidden energy gap between the valence band and conduction band is zero meaning the valence and conduction band is overlapped.
While in semiconductor, the forbidden energy gap between the valence band and conduction band is less. Therefore conductivity of semiconductors lies between the insulator and conductor. Examples of semiconductors are Silicon, germanium, arsenic, etc. The forbidden gap in a semiconductor is of the order of kT. At very low temperatures, the valence band is completely occupied by the valence electron and the conduction band is completely empty because no sufficient energy is present in the valence electron to jump into the conduction band. Therefore the forbidden energy gap remains the same as in insulators. Hence conduction won’t be possible and therefore the semiconductor acts as an insulator.
When the temperature increases, the top level of valence electron from valence electron gains some thermal energy i.e. kT greater than forbidden energy Eg. Hence a valence electron becomes a free electron and jumps into the conduction band which is responsible for the decrease in the forbidden energy gap and leads to conduction.
After the ejection of an electron from the valence band, a deficiency of electron is generated. It is known as a hole and acts as a positive charge. Thus the transfer of an electron from the valence to conduction creates an equal number of holes in the valence band and an equal number of electrons in the conduction band. These holes also cause decrees in the gap and hence conduction. Therefore as the temperature gets increases, more and more free electrons would be generated and this will cause, decrease in the forbidden energy gap between the valence band and conduction band.
Therefore, with an increase in temperature of a semiconductor its conductivity increases.
Note: Semiconductors have a negative temperature coefficient i.e. resistance of semiconductor decreases with an increase in temperature. The addition of suitable metallic impurity causes a change in conductivity. In the case of extrinsic semiconductors, the number of majority carriers is nearly constant, but mobility decreases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




