
write bond line formula of: isopropyl alcohol
Answer
585.9k+ views
Hint: Bond-line structure (bond-line formula, skeletal structure, skeletal formula): A representation of molecular structure in which covalent bonds are represented with one line for each level of bond order. Bond-line structural representation of Taxol, an anticancer drug.
Complete step by step answer:
We have been provided with a compound: isopropyl alcohol,
We need to write its bond line formula,
So, for that firstly: Isopropyl alcohol is a colourless, flammable chemical compound with a strong odour. As an isopropyl group linked to a hydroxyl group, it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of 1-propanol and ethyl methyl ether.
We know that, to draw the bond line formula of a compound, first of all we have to draw the complete structure of each compound and then convert it into a bond line formula in which the joint represents the carbon atoms.
So, the bond line formula of isopropyl alcohol is:
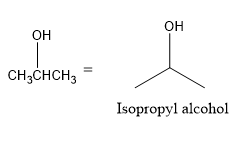
Isopropyl alcohol is readily absorbed through the skin, so spilling large amounts of IPA on the skin may cause accidental poisoning. Small amounts of IPA on the skin is generally not dangerous, but repeated skin exposure can cause itching, redness, rash, drying, and cracking. Prolonged skin contact may cause corrosion. So, therefore these are the bond line structures and formula of Isopropyl alcohol.
Note: Isopropyl alcohol is mixed with water for use as a rubbing-alcohol antiseptic. It is also used in aftershave lotions, hand lotions, and other cosmetics. In industry it is used as an inexpensive solvent for cosmetics, drugs, shellacs, and gums, as well as for denaturing ethanol (ethyl alcohol).
Complete step by step answer:
We have been provided with a compound: isopropyl alcohol,
We need to write its bond line formula,
So, for that firstly: Isopropyl alcohol is a colourless, flammable chemical compound with a strong odour. As an isopropyl group linked to a hydroxyl group, it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of 1-propanol and ethyl methyl ether.
We know that, to draw the bond line formula of a compound, first of all we have to draw the complete structure of each compound and then convert it into a bond line formula in which the joint represents the carbon atoms.
So, the bond line formula of isopropyl alcohol is:

Isopropyl alcohol is readily absorbed through the skin, so spilling large amounts of IPA on the skin may cause accidental poisoning. Small amounts of IPA on the skin is generally not dangerous, but repeated skin exposure can cause itching, redness, rash, drying, and cracking. Prolonged skin contact may cause corrosion. So, therefore these are the bond line structures and formula of Isopropyl alcohol.
Note: Isopropyl alcohol is mixed with water for use as a rubbing-alcohol antiseptic. It is also used in aftershave lotions, hand lotions, and other cosmetics. In industry it is used as an inexpensive solvent for cosmetics, drugs, shellacs, and gums, as well as for denaturing ethanol (ethyl alcohol).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




