
Write the chemical reaction of chloroform with the following:
(1) Silver Powder
(2) Concentrate $HN{O_3}$
(3) Acetone
(4) Alkaline Solution of phenol
Answer
582.6k+ views
Hint: Chloroform is an organic compound when inhaled it causes dizziness fatigue and headache moreover direct exposure of it in air sunlight is avoided.
We know that chloroform is an organic compound with cervical formula $CHC{l_3}$ and structure as.

Complete step by step answer:
Chloroform is a colorless, strong smelling and dense liquid. Earlier before chloroform is used as anesthetic, A patient is forced to smell the chloroform before any operation/surgery. But now-a-day use of chloroforms as an anesthetic is decreased now-a-days because of oxidation in air and sunlight, it gives a harmful poisonous gas phosgene that can cause death of person.
$CHC{l_3} + \dfrac{1}{2}{O_2}\xrightarrow{{sunlight}}COC{l_2} + HCl$
Thus, chloroform is stored in dark cultured bottles [to cut off sunlight] completely filled to the brim (to beep air out).
Now we have to write resections of chloroform with compounds given is the question.
1.Reaction with silver powder when chloroform is reacted with silver powder, it causes DE halogenation and bonds to the formation of acetylene.
$2CHC{l_3} + 6Ag \to {C_2}{H_2} + 6AgCl$
2.Reaction with cone $HN{O_3}$no treating chloroform with concentrators leads to the formation of nitro chloroform [are chloropicrin] it is a poisonous gas.
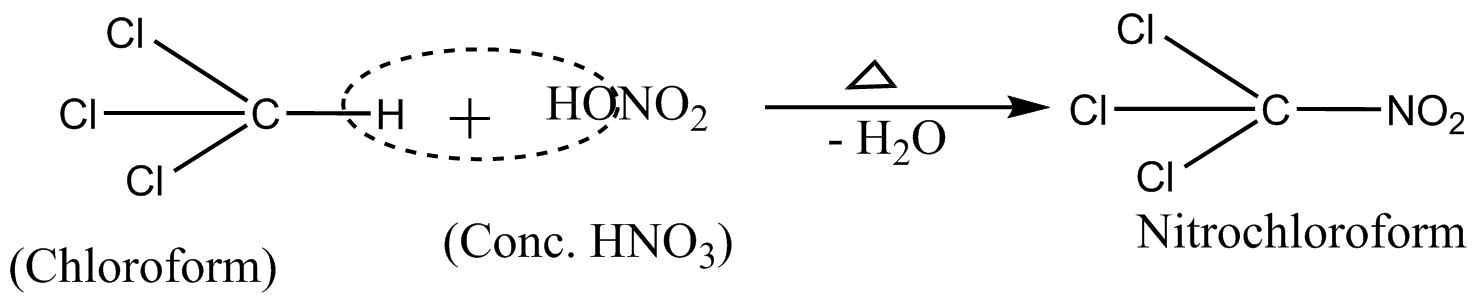
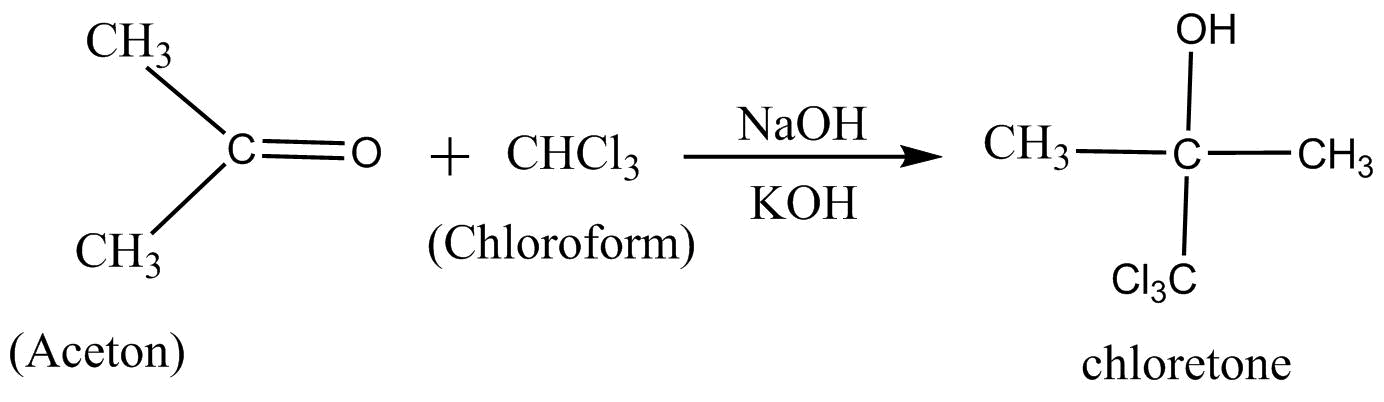
3.Reaction with acetone chloroform reacts with acetone $\left[ {C{H_3} - C - C{H_3}} \right]$ in presence of sodium hydroxide to produce high grade drug sleep chlorate.
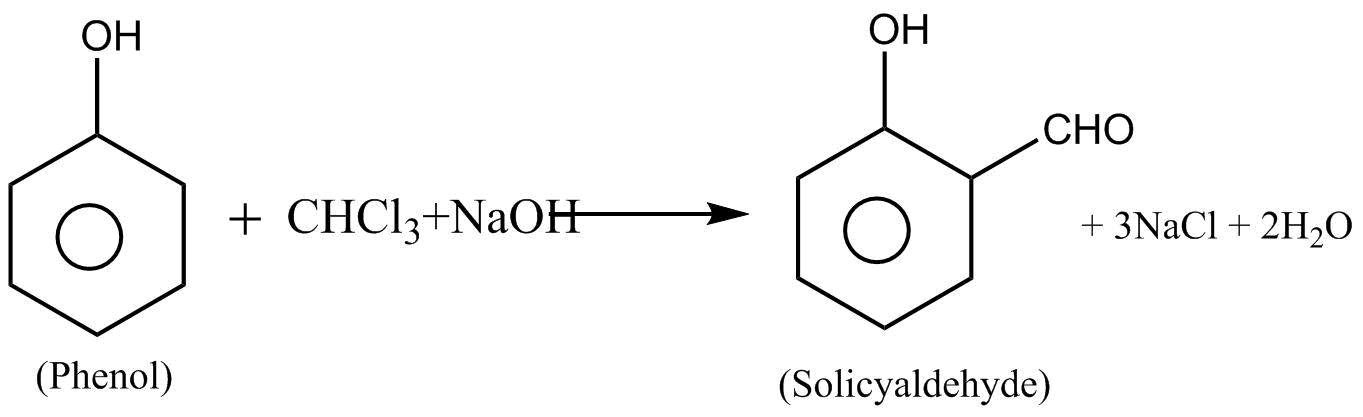
4.Reaction with alkaline solution of phenol:-
When chloroform is treated with an alkaline solution of phenol to yield salicylaldehyde as the main product. This reaction is also known as rime Tomean reaction.
Note:
The alkaline solution of phenol means that phenol is clussolued in basic so went. Moreover a little of 1% of ethanol added chladorarm bottle which converts harmful phosgene gas into harmless clothe carbonate.
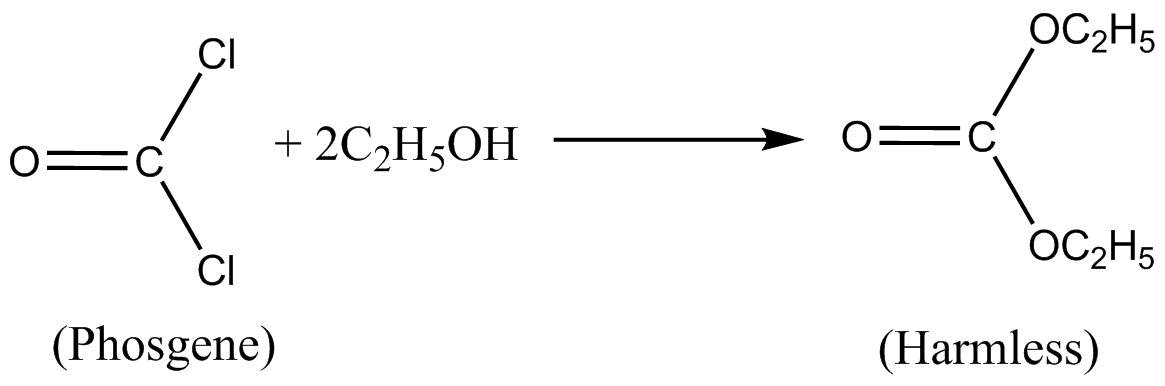
We know that chloroform is an organic compound with cervical formula $CHC{l_3}$ and structure as.

Complete step by step answer:
Chloroform is a colorless, strong smelling and dense liquid. Earlier before chloroform is used as anesthetic, A patient is forced to smell the chloroform before any operation/surgery. But now-a-day use of chloroforms as an anesthetic is decreased now-a-days because of oxidation in air and sunlight, it gives a harmful poisonous gas phosgene that can cause death of person.
$CHC{l_3} + \dfrac{1}{2}{O_2}\xrightarrow{{sunlight}}COC{l_2} + HCl$
Thus, chloroform is stored in dark cultured bottles [to cut off sunlight] completely filled to the brim (to beep air out).
Now we have to write resections of chloroform with compounds given is the question.
1.Reaction with silver powder when chloroform is reacted with silver powder, it causes DE halogenation and bonds to the formation of acetylene.
$2CHC{l_3} + 6Ag \to {C_2}{H_2} + 6AgCl$
2.Reaction with cone $HN{O_3}$no treating chloroform with concentrators leads to the formation of nitro chloroform [are chloropicrin] it is a poisonous gas.
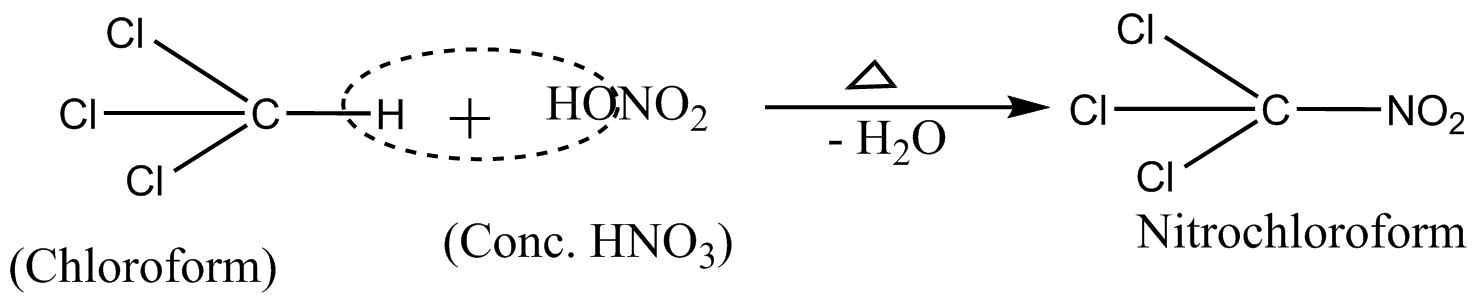
3.Reaction with acetone chloroform reacts with acetone $\left[ {C{H_3} - C - C{H_3}} \right]$ in presence of sodium hydroxide to produce high grade drug sleep chlorate.

4.Reaction with alkaline solution of phenol:-
When chloroform is treated with an alkaline solution of phenol to yield salicylaldehyde as the main product. This reaction is also known as rime Tomean reaction.

Note:
The alkaline solution of phenol means that phenol is clussolued in basic so went. Moreover a little of 1% of ethanol added chladorarm bottle which converts harmful phosgene gas into harmless clothe carbonate.
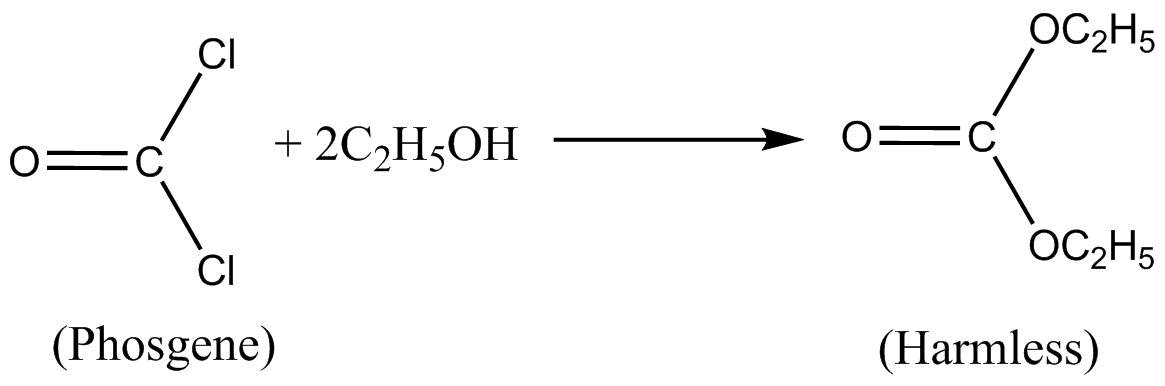
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




