
The C — H bond and C— C bond in ethane are formed by which of the following
types of overlap?
(a)- \[s{{p}^{3}}-s\] and \[s{{p}^{3}}-s{{p}^{3}}\]
(b)- \[s{{p}^{2}}-s\] and \[s{{p}^{2}}-s{{p}^{2}}\]
(c)- \[s{{p}^{2}}-s\] and \[sp-sp\]
(d)- \[p-s\]and \[p-p\]
Answer
233.1k+ views
Hint:Covalent bonds are defined as the bonds formed due to sharing of electrons or we can say that they bond through overlapping of atomic orbitals of participant atoms. A covalent bond prefers specific orientations in space.
Complete step by step solution:
> Sigma(σ) bonds can be formed by any one of the following types of combinations of atomic orbitals.
- s-s overlapping: Overlap of two half-filled s-orbitals along the internuclear axis.
- s-p overlapping: Overlap between half-filled s-orbitals of one atom and half-filled p-orbitals of another atom.
- p–p overlapping: Overlap between half-filled p-orbitals of the two approaching atoms.
> First let’s draw the structure of ethane molecules.
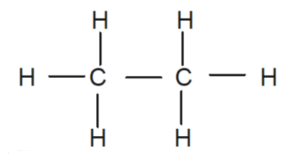
> From the structure we can see each carbon forms four bonds, three bonds with a Hydrogen atom and one with the adjacent carbon atom. To find the hybridization of the molecule, first we will isolate both of the Carbon atoms and write down their electron configuration, which is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}\]. From the electronic configuration we can see that only two unpaired electrons are available in orbit but we know that carbon can form four bonds, making it obvious that hybridization is required to make the four unpaired electrons available for this bonding. As a result, four \[s{{p}^{3}}\] hybrid orbitals are formed on each carbon.
> Now, both of the carbons will have four bonds arranged in tetrahedral geometry. The carbon-carbon sigma bond is formed by overlapping of the \[s{{p}^{3}}\] hybrid orbital of the one carbon with the other carbon. which also has three Hydrogens bonded to it in the similar manner., while the six carbon-hydrogen sigma bonds are formed from overlaps between the \[s{{p}^{3}}\] orbitals on the two carbons and the 1s orbitals of six hydrogen
atoms. And we get a molecule with a total of seven sigma bonds and eventually the ethane molecule.
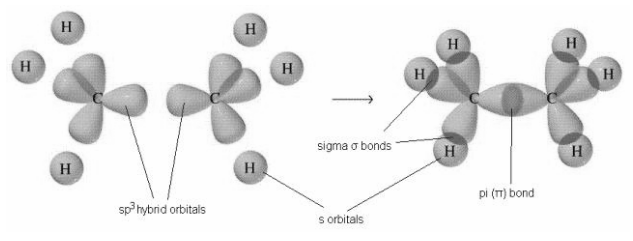
So, the correct option is (a).
Note: C–C bond length is 154 pm and each C–H bond length is 109 pm. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. In this case of ethane molecules, the two methyl (\[C{{H}_{3}}\]) groups are free to rotate.
Complete step by step solution:
> Sigma(σ) bonds can be formed by any one of the following types of combinations of atomic orbitals.
- s-s overlapping: Overlap of two half-filled s-orbitals along the internuclear axis.
- s-p overlapping: Overlap between half-filled s-orbitals of one atom and half-filled p-orbitals of another atom.
- p–p overlapping: Overlap between half-filled p-orbitals of the two approaching atoms.
> First let’s draw the structure of ethane molecules.

> From the structure we can see each carbon forms four bonds, three bonds with a Hydrogen atom and one with the adjacent carbon atom. To find the hybridization of the molecule, first we will isolate both of the Carbon atoms and write down their electron configuration, which is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}\]. From the electronic configuration we can see that only two unpaired electrons are available in orbit but we know that carbon can form four bonds, making it obvious that hybridization is required to make the four unpaired electrons available for this bonding. As a result, four \[s{{p}^{3}}\] hybrid orbitals are formed on each carbon.
> Now, both of the carbons will have four bonds arranged in tetrahedral geometry. The carbon-carbon sigma bond is formed by overlapping of the \[s{{p}^{3}}\] hybrid orbital of the one carbon with the other carbon. which also has three Hydrogens bonded to it in the similar manner., while the six carbon-hydrogen sigma bonds are formed from overlaps between the \[s{{p}^{3}}\] orbitals on the two carbons and the 1s orbitals of six hydrogen
atoms. And we get a molecule with a total of seven sigma bonds and eventually the ethane molecule.

So, the correct option is (a).
Note: C–C bond length is 154 pm and each C–H bond length is 109 pm. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. In this case of ethane molecules, the two methyl (\[C{{H}_{3}}\]) groups are free to rotate.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




