
Which one of the following about atomic structure is FALSE?
A.The electrons occupy a very large volume compared to the nucleus.
B.The number of protons and electrons in a neutral atom is the same.
C.The number of electrons in an atom can be found by subtracting the atomic number of an element from the mass number of an element.
D.Almost all of the mass of an atom is found in the nucleus.
Answer
233.1k+ views
Hint: Atomic structure basically refers to the structure of an atom consisting of a nucleus in which the protons and neutrons are present. Moreover, there are negatively charged particles known as electrons which revolve around the center of the nucleus.
Complete step by step answer:
The first scientific theory of atomic structure was proposed by John Dalton in. The atomic structure of an element refers to the constitution of its nucleus and the arrangement of electrons around it. It is basically made up of protons, electrons and neutrons. Further, John Dalton suggested that all matter is made up of atoms which are indivisible and indestructible. Some of the postulated of his theory were:
1.Every matter is made up of atoms
2.Atoms undergo rearrangement during a chemical reaction
3.Each atom has its own constant mass that varies from element to element.
4.Atoms are indivisible
The structure of atom is as shown:
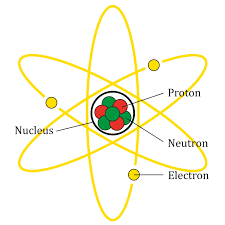
Now, let’s consider each option and find out the false one.
So, option A says that electrons occupy a large volume as compared to the nucleus. This statement is true as nucleus takes up very little space and the electron cloud is generally times bigger than the nucleus.
Further, Option B says that the number of protons and neutrons in a neutral atom are same. This statement is also true because if there were more protons or more electrons than the atom would develop a charge and thus will not be neutral.
Option C say that the difference between mass number and the atomic number should be equal to the number of protons. This statement is false because this value gives the number of neutrons.
Further, the last option is true because electrons have very small mass and therefore most of the mass of the atom is found inside the nucleus.
Hence, option C is correct.
Note:In and centuries, many scientists attempted to explain the structure of the atom with the help of atomic models. But the most notable contributions to this field were by the scientists John Dalton, J.J. Thomson, Ernest Rutherford and Niels Bohr.
Complete step by step answer:
The first scientific theory of atomic structure was proposed by John Dalton in. The atomic structure of an element refers to the constitution of its nucleus and the arrangement of electrons around it. It is basically made up of protons, electrons and neutrons. Further, John Dalton suggested that all matter is made up of atoms which are indivisible and indestructible. Some of the postulated of his theory were:
1.Every matter is made up of atoms
2.Atoms undergo rearrangement during a chemical reaction
3.Each atom has its own constant mass that varies from element to element.
4.Atoms are indivisible
The structure of atom is as shown:

Now, let’s consider each option and find out the false one.
So, option A says that electrons occupy a large volume as compared to the nucleus. This statement is true as nucleus takes up very little space and the electron cloud is generally times bigger than the nucleus.
Further, Option B says that the number of protons and neutrons in a neutral atom are same. This statement is also true because if there were more protons or more electrons than the atom would develop a charge and thus will not be neutral.
Option C say that the difference between mass number and the atomic number should be equal to the number of protons. This statement is false because this value gives the number of neutrons.
Further, the last option is true because electrons have very small mass and therefore most of the mass of the atom is found inside the nucleus.
Hence, option C is correct.
Note:In and centuries, many scientists attempted to explain the structure of the atom with the help of atomic models. But the most notable contributions to this field were by the scientists John Dalton, J.J. Thomson, Ernest Rutherford and Niels Bohr.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Difference Between Crystalline and Amorphous Solid: Table & Examples

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

In Carius method of estimation of halogens 015g of class 11 chemistry JEE_Main

Understanding How a Current Loop Acts as a Magnetic Dipole

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding Atomic Structure for Beginners

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 1 Some Basic Concepts of Chemistry (2025-26)

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 8 Redox Reactions (2025-26)




