Revision Notes on Surface Chemistry for NEET 2026 - Free PDF Download
Students who are in class 12 and learning Chemistry will come over to the subject of Surface Chemistry. The Class 12 Chemistry Surface Chemistry Notes cover the entire chapter having information on Adsorption, Adsorbate, Desorption, and others. Students will also learn about Types of Adsorption and factors affecting the adsorption of gases on solids. Using the Surface Chemistry NEET Notes, students can prepare well for the exam as a part of last-minute revision.
These Surface Chemistry Class 12 Notes are prepared by subject matter experts of Vedantu, presenting each topic in simple language. Students can download Surface Chemistry Notes PDF for free and practice the chapter anytime.
NEET Revision Notes Chemistry Surface Chemistry
General Introduction
Surface chemistry is the discipline of chemistry that studies events that occur at the surface or interface, that is, at the boundary separating two bulk phases.
Pure chemicals or solutions can make up the two bulk phases.
A hyphen or a slash between the two bulk phases involved, such as solid-liquid or solid/liquid, is used to signify the interface. Because gases are entirely miscible, there is no interface between them. Dissolution, crystallization, corrosion, heterogeneous catalysis, electrode processes, and other important phenomena occur at the interface.
Adsorption
It is the process of attracting and holding a substance's molecules on the surface of a liquid or solid, resulting in a larger concentration of molecules on the surface. Occlusion is the process of gases adsorbing on a metal surface.
Adsorbate and Adsorbent
Adsorbate refers to a material that is adsorbed on any surface. For example, if gas is adsorbed on the surface of a solid, the gas is referred to as the adsorbate.
The adsorbent is the substance on the surface of which adsorption occurs.
The adsorbent might be either solid or liquid. Metal powders, powdered charcoal, animal charcoal, silica powder, and other adsorbents are often employed.
Desorption
Desorption is the process of removing an adsorbed material from a surface. This can be accomplished by increasing or decreasing the system's pressure.
Absorption
The phenomenon of absorption occurs when the molecules of a substance are consistently distributed throughout the body of a solid or liquid.
Sorption
It is a phenomenon in which adsorption and absorption occur at the same time. Cotton fiber absorbs dyes just as well as other fibers.
Adsorption is a fast, instantaneous process, whereas absorption is a lengthy process.
Difference Between Adsorption and Absorption
The following are the main points of distinction between adsorption and absorption.
Adsorption | Absorption |
It's a purely physical phenomenon. | It is concerned with the absorbent's entire mass. |
The substance is only kept on the surface and does not penetrate in the solid or liquid bulk or interior. | It denotes that a component is evenly distributed throughout the solid or liquid's body. |
In the free phase, the concentration of adsorbed molecules is always higher. | It has a low concentration. |
It starts off fast and then slows down to reach equilibrium. | It happens at a constant rate. |
Examples (i) \[{\text{CaC}}{{\text{l}}_{\text{2}}}\] adsorbs water vapours in the first place. (ii) Charcoal adsorbs ammonia. (iii) Activated or animal charcoal is used to decolorize sugar solutions. | Examples (i) Anhydrous silica gel absorbs water vapors; (ii) \[{\text{N}}{{\text{H}}_3}\] is absorbed in water and forms \[{\text{N}}{{\text{H}}_4}{\text{OH}}\] |
Mechanism of Adsorption
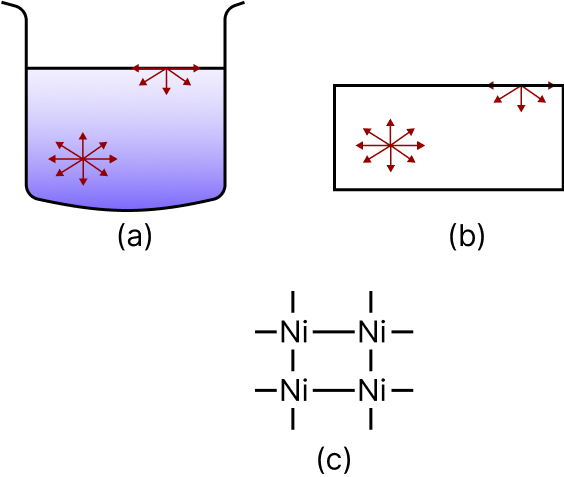
Different Types of Matter
In the case of (a) liquid (b) solid (c) metal with unbound valencies, molecules at the surface experience a net inward force of attraction.
Adsorption occurs only when an adsorbent's surface atoms are active. Unbalanced forces of various types, such as Van der Waal's forces and chemical bond forces.
Liquids and solids can attract and keep the molecules of a gas or dissolved material on their surface due to residual or imbalanced inward forces of attraction at the surface.
Characteristics of Adsorption
The presence of a larger concentration of any given component at the surface of a liquid or solid phase is referred to as adsorption.
Adsorption is always followed by the release of heat, making it an exothermic reaction. To put it another way, the adsorption H is always negative.
When a gas is adsorbed, the molecules' freedom of movement is restricted. As a result, the entropy of the gas after adsorption decreases, i.e. S is negative.
Adsorption is consequently accompanied by a decrease in the system's enthalpy as well as entropy, and hence G decreases.
The thermodynamic criteria for a process to be spontaneous is that G must be negative.
Classification of Adsorption
Adsorption is divided into two groups, they are:
Physical Adsorption: Physical adsorption occurs when the forces of attraction between the adsorbate and the adsorbent are Van der Waal's forces.
Chemical Adsorption: Chemical adsorption occurs when the forces of attraction between adsorbate particles and the adsorbent are almost equal in strength to chemical bonds.
Comparison Between Physisorption and Chemisorption
Physisorption (Van der Waal’s adsorption) | Chemisorption (Langmuir adsorption) |
Adsorption heat is normally in the 20-40 kJ/mol range. | 1. Adsorption heat in the range of 50-400 kJ mol. |
Van der Waal's forces are forces of attraction. | Chemical bond forces are forces of attraction. |
It's reversible. | It is irreversible. |
It frequently occurs at low temperatures and reduces as the temperature rises. | It takes place preferably in a hot environment. |
It does not require the use of an appreciable amount of activation energy. | It requires a large amount of activation energy. |
High pressure is advantageous. Desorption occurs when the pressure drops. | High pressure is advantageous. Desorption is not caused by a decrease in pressure. |
It lacks specificity. | It's quite particular. |
Note: Physical adsorption reduces after a period due to the creation of multilayers.
Both chemisorption and physisorption are exothermic processes.
Aspects that Influence the Amount of Adsorption on a Solid Surface
The following are some of the elements that influence gas adsorption on solid surfaces.
(i) In comparison to hard non-porous materials, porous and finely powdered solids, such as charcoal and fuller earth, absorb more. Powdered charcoal is utilized in coal mine gas masks because of this feature.
(ii) When compared to gases with lower critical temperatures, gases with higher critical temperatures are adsorbed to a greater extent.
Positive and Negative Adsorption
Positive adsorption occurs when the concentration of the adsorbate is higher on the surface of the adsorbent than in the bulk. Negative adsorption occurs when the concentration of the adsorbate in the bulk increases after adsorption.
Blood solution + Conc. \[{\text{KCl}}\] solution \[ \to \] Positive adsorption
Blood solution + dilute \[{\text{KCl}}\] solution \[ \to \] Negative adsorption
Applications of Adsorption
The adsorption phenomenon has a wide range of uses. The following are some of the most important applications.
Production of High Vacuum:
A bulk of charcoal that has been cooled in liquid air is attached to a vessel that has already been vacuumed as much as feasible. The remaining air is absorbed by the charcoal, resulting in an extremely high vacuum.
In the Case of Gas Masks: It's an adsorbent device made of activated charcoal or a combination of adsorbents. In coal mines, this system is used to adsorb harmful gases (e.g. \[{\text{C}}{{\text{l}}_2}\], \[{\text{CO}}\], sulphur oxide, etc.) and so purify the air for breathing.
Desiccation or Dehumidification: Certain substances, such as silica and alumina $Al_{2}O_{3}$, have a significant tendency to absorb water. Water vapours or moisture in the air can be reduced or removed using these compounds.
In electrical equipment, silica gel is also utilised for dehumidification.
Color Removal From Solutions: Animal charcoal adsorbs coloured impurities to remove the colour from solutions. It's also employed in the production of cane sugar as a decolourizer.
Separation of Inert Gases: A mixture of inert gases can be separated by adsorption on coconut charcoal at different low temperatures due to the difference in the degree of adsorption of gases by charcoal.
Hard Water Softening: The hard water is forced to travel through a zeolite-filled column (sodium aluminium silicate)
Chromatographic Analysis: The phenomena of adsorption has given rise to chromatographic analysis, a good technique of analysis. The method has a wide range of applications in both analytical and industrial settings.
Types of Catalysis
The following types of catalytic reactions can be generally classified:
1. When the reactants and catalyst are in the same phase, it is called homogeneous catalysis (i.e. solid, liquid or gas). It is claimed that the catalysis is homogenous. An example of homogeneous catalysis is listed below.
Sugar hydrolysis is catalysed by H+ ions produced by sulphuric acid.
\[{{\text{C}}_{12}}{{\text{H}}_{22}}{{\text{O}}_{11}}{\text{(}}aq {\text{) + }}{{\text{H}}_2}{\text{O(}}l {\text{)}}\xrightarrow{{{{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(l)}}}}{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_6}{\text{(}}aq {\text{) + }}{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_6}{\text{(}}aq {\text{) }}\]
Mixture of Glucose and Fructose formed in above reaction.
2. Heterogeneous catalysis refers to a catalytic process in which the reactants and catalyst are in different phases at the same time. The following are some examples of heterogeneous catalysis.
In the contact process for the synthesis of sulphuric acid, oxidation of sulphur dioxide into sulphur trioxide in the presence of platinum metal or vanadium pentaoxide as catalyst. The reactants are in gaseous form, while the catalyst is solid.
\[2{\text{S}}{{\text{O}}_2}(g) + {O_2(g)}\xrightarrow{{{\text{Pt(s)}}}}2{\text{S}}{{\text{O}}_3}(g)\]
In Haber's method, nitrogen and hydrogen combine to generate ammonia in the presence of finely split iron.
\[{{\text{N}}_2}(g) + 3{{\text{H}}_2}(g)\xrightarrow{{Fe(s)}}2{\text{N}}{{\text{H}}_3}(g)\]
In Ostwald's procedure, ammonia is converted to nitric oxide in the presence of platinum gauze as a catalyst.
\[{\text{4N}}{{\text{H}}_3}{\text{(g)}} + 5{{\text{O}}_2}{\text{(g)}}\xrightarrow{{Pt(s)}}{\text{4NO(g)}} + 6{{\text{H}}_2}{\text{O(g)}}\]
Vegetable oils are hydrogenated in the presence of finely divided nickel as a catalyst.
\[{\text{Vegetable oils}}\left( {\text{l}} \right){\text{ + }}{{\text{H}}_{\text{2}}}\left( {\text{g}} \right){\text{ }}\xrightarrow{{{\text{Ni}}\left( {\text{s}} \right)}}{\text{Vegetable Ghee}}\left( {\text{g}} \right)\]
Characteristics of Catalysis
The following are the properties that most catalytic reactions have in common.
At the end of the reaction, the mass and chemical composition of a catalyst remain unaltered.
The catalyst cannot start the reaction: The catalyst's role is to change the rate of the reaction rather than to initiate it.
The catalyst is usually very specific: A catalyst is a material that speeds up the rate of a process.
Different catalysts for the same reactant may produce different products if they fail to catalyze the other reaction.
Catalytic poisons: Catalytic poisons are substances that, simply by being present, disrupt the catalyst's activity.
Enzyme Catalysis
All forms of plants and organisms secrete enzymes, which are complex nitrogenous compounds. Protein molecules with a larger molecular mass are known as enzymes. (between 15,000 and 1,000,000 g/mol) Enzymes are particularly good catalysts because they produce colloidal solutions in water. Enzymes are responsible for catalyzing these processes. As a result, enzymes are referred to as biochemical catalysts, and the phenomenon is referred to as biochemical catalysis.
Activity and Selectivity
Activity: Activity refers to a catalyst's ability to speed up chemical reactions. For example, in the presence of platinum as a catalyst, the reaction between \[{{\text{H}}_2}\] and \[{{\text{O}}_2}\] to create H2O. \[{{\text{H}}_2}\] and \[{{\text{O}}_2}\] can be held indefinitely without undergoing any reactions in the absence of a catalyst. Pt provides a surface for gases to get adsorbed.
Selectivity: Catalysts' ability to control reactions to produce certain products (excluding others).
Zeolites (shape selective catalysis)
Zeolites are alumino–silicates. They have a cage that looks like a honeycomb. The shape and size of the reactant and product molecules influence the processes catalysed by zeolites. Therefore, these reactions are referred to as shape selective catalysis.
Colloidal State: The size of the particles determines the colloidal condition. It's thought to be a halfway point between full solution and suspension. Their range of diameters is between 1 and 1000 nm.
1. True solution: Because the size of the particles of solute in real solutions is so minute that they can't be recognised by any optical means and freely diffuse through membranes, they can't be detected by any optical methods. It's a one-of-a-kind system.
2. Suspension: Particles are large enough to be seen with the naked eye and do not pass-through filter paper. It's a complicated system.
Characteristics of True solutions, Colloidal Solutions and Suspensions
S.No | Property | True Solutions | Colloidal Solutions | Suspensions |
1 | Nature | Homogeneous | Heterogeneous | Heterogeneous |
2 | Particle size (diameters) | Less than 10–9 m or 1 nm (i.e., < 10 Å) | Between 10–9 to 10–6m or 1 nm to 1000 nm | More than 10–6m or 1000 nm (i.e., > 10000Å |
3 | Filterability | Pass through regular filter paper. | Some semipermeable membranes will not pass-through but regular filter paper will. | Filter paper can separate it easily. |
4 | Settling | Don't settle. | Don't settle. | Settle down. |
5 | Visibility | Particles are imperceptible to the naked eye. | Under an ultra-microscope, light scattering by the particles are observed. | Particles can be seen with the naked eye or via a microscope. |
6 | Diffusion | Diffusion is quick | Diffusion is slow | Diffusion does not take place |
7 | Appearance | transparent and clear | Translucent | Opaque |
Dispersed Phase and Dispersion Medium
Dispersed Phase (Discontinuous Phase): This is a component that exists in small amounts and behaves similarly to a solute in a solution. For instance, in a colloidal silver solution in water (silver acts as a dispersed phase)
Dispersion Medium (Continuous Phase): This is the component that is usually present in excess and functions similarly to a solvent in a solution. For instance, in a colloidal silver solution in water. The dispersion medium is water.
Classification of Colloids
Dispersion Phase and Dispersion Medium Classification based on Physical State
There are eight different types of colloidal systems depending on the physical state of the dispersed phase and the dispersion medium, which might be solids, liquids, or gases.
Different Types of Colloidal System
Sr. No | Dispersed Phase | Dispersion | Colloidal System | Examples |
1 | Liquid | Gas | Aerosol of liquids | Fine pesticide sprays, fogs, clouds, and mists |
2 | Solid | Gas | Aerosol of solid | Smoke, dust |
3 | Gas | Liquid | frothy lemonade, foam, whipped cream, and soda water | |
4 | Liquid | Liquid | Emulsions | Medicines, emulsified oils, and milk |
5 | Solid | Liquid | Sols | Most paints, water-soluble starch, proteins, gold sol, arsenic sulfide sol, and ink |
6 | Gas | Solid | Solid foam | Styrene rubber, pumice stone, and foam rubber |
7 | Liquid | Solid | Gels | Butter, cheese, boot polish, jelly |
8 | Solid | Solid | Solid sols (colored glass) | Some gemstones and alloys, as well as ruby glass |
Classification Based On the Nature of the Dispersed Phase's Interaction With the Dispersion Medium:
liquid-loving colloids are known as lyophilic colloids. "Lyophilic colloids are colloidal solutions in which the particles of the dispersed phase have a strong affinity for the dispersion medium."
"The colloidal solutions in which there is no affinity between particles of the dispersed phase and the dispersion medium are called lyophobic colloids."
Distinction Between Lyophilic and Lyophobic Sols
Sr. No | Property | Lyophilic (suspension) | Lyophobic Sols (Emulsoid) |
1 | Viscosity | It is much higher than the medium. | Identical to that of the medium |
2 | Reversibility | Reversible | Irreversible |
3 | Stability | More stable | Less stable |
4 | Visibility | Even under an ultra-microscope, particles are undetectable. | Under an ultra-microscope, particles can be seen. |
Classification Based on Dispersed Phase Particle Types:
The colloids are categorized as follows based on the sort of particles in the dispersed phase.
Colloids are made up of several molecules. Multimolecular colloids are generated when atoms or smaller molecules of substances gather together during dissolution to form particles of colloidal size.
The dispersed phase in these sols is made up of clumps of atoms or molecules with a molecular size of around 1 nm.
Sols of gold atoms and sulphur molecules, for example. Van der Waals forces hold the particles together in these colloids. They are typically lyophilic in nature.
Macromolecular colloids: These are substances with large molecules (called macromolecules) that dissolve to produce colloidal particles. Macromolecular colloids are the name given to such things.
These distributed macromolecules are often polymers with extremely large molecular masses.
Starch, cellulose, proteins, enzymes, gelatin, and other naturally occurring macromolecules are examples.
Associated Colloids are chemicals that, when dissolved in a medium, behave as conventional electrolytes at low concentrations but, due to the production of aggregated particles, behave as colloidal particles at larger concentrations.
Micelles are the aggregated particles that result from this process.
Both lyophilic and lyophobic groups can be found in their molecules.
Properties of Colloidal Solutions
The following are the main characteristics of colloidal solutions.
Physical Characteristics:
Nature of Colloidal Sols: Colloidal sols are heterogeneous by nature. The Dispersed Phase and the Dispersion Medium are the two phases.
The colloidal solutions have a very stable character. Their particles are in a state of motion and do not settle to the container's bottom.
Filterability: Colloidal particles flow through standard filter sheets with ease. Special filters known as ultrafilters can, however, keep them out (parchment paper).
Colligative Properties:
The observed values of colligative qualities such as the relative decrease in vapor pressure, elevation in boiling point, depression in freezing point, and osmotic pressure are smaller than expected due to the creation of linked molecules.
In comparison to the real solution, the number of particles in a colloidal sol will be very small.
Mechanical Properties:
Brownian Movement: The colloidal particles are moving in a zig-zag pattern at random. Brownian motion is the name for this sort of motion.
Brownian Motion is caused by the molecules of the dispersion medium colliding with the particles of the dispersed phase on a regular basis.
Diffusion: Sol particles diffuse from a region of higher concentration to a region of lower concentration. However, because of their larger size, they diffuse at a slower rate.
Sedimentation: Under the action of gravity, colloidal particles settle down at a very slow rate. The molecular mass of macromolecules is determined using this phenomenon.
Optical Properties: Tyndall Effect:
Because light is scattered by particles as it passes through a sol, its path becomes apparent. It's known as the Tyndall effect.
Tyndall was the first to investigate this phenomenon. Tyndall cone refers to the lit path of the beam. There are no particles with a big enough diameter to scatter light in a real solution, hence there is no Tyndall effect.
A device known as an ultra–microscope has also been used to investigate the Tyndall phenomenon.
Electrical Properties: Colloidal particles have an electric charge, whereas the dispersion medium has an equal and opposite charge, making the system electrically neutral. Because mutual forces of repulsion between similarly charged particles prevent them from coalescing and coagulating when they get closer to one another, the existence of equal and similar charges on colloidal particles is largely responsible for the system's stability.
Electrophoresis
Electrophoresis is the movement of colloidal particles in the presence of an electric field. The charge on the particles is positive if they collect near the negative electrode. The charge on the sol particles, on the other hand, is negative if they collect near the positive electrode.
Electrical Double-layer Theory:
Electrical double-layer theory can also be used to describe the electrical properties of colloids. A double layer of ions appears at the surface of a solid, according to this idea.
The ion that is preferentially adsorbed is held in a fixed portion and gives colloidal particles a charge.
A diffused mobile ion layer makes up the second component. Both types of charges are present in the second layer.
When an electric field is used, the particles now move (electrophoresis).
Electro-osmosis:
In this process, a semipermeable membrane prevents scattered particles from migrating.
The phenomenon of electro-osmosis occurs when a dispersion medium is permitted to move under the influence of an electrical field while colloidal particles are not allowed to move.
Coagulation or Flocculation or Precipitation
"The phenomena of coagulation or flocculation is the precipitation of a colloidal solution by the addition of an excess of an electrolyte."
Electrophoresis (electrophoresis): Colloidal particles travel towards the oppositely charged electrode in electrophoresis. These are released and precipitated when they come into prolonged contact with the electrode.
By Combining Two Sols with Opposite Charges: The charges of oppositely charged sols are neutralized when they are blended in almost equal proportions. Because ferric hydroxide (+ve sol) and arsenious sulfide (–ve sol) are mixed, both sols can be partially or totally precipitated.
When a sol is boiled, the adsorbed layer is disrupted due to increased collisions with the dispersion medium molecules. The charge on the particles is reduced, and they eventually settle down to form a precipitate.
By long-term Dialysis: Long-term dialysis removes practically all traces of the electrolyte contained in the sol, making the colloids unstable.
By Adding Electrolytes: The dispersed phase particles, i.e. colloids, have a charge. When an electrolyte is put to a sol, the colloidal particles absorb ions from the electrolyte that have the opposite charge. As a result, their charge is neutralized, causing the uncharged particles to clump together and coagulate or precipitate. When \[{\text{BaC}}{{\text{l}}_2}\] solution is added to \[{\text{A}}{{\text{s}}_2}{{\text{S}}_3}\] sol, for example, the \[{\text{B}}{{\text{a}}^{2 + }}\] ions are attracted to the negatively charged sol particles and their charge is neutralised. Coagulation occurs as a result of this.
Coagulation or Flocculation Value
"Flocculation value refers to the minimal concentration of an electrolyte required to cause coagulation or flocculation of a sol."
or "The flocculation value is the number of millimoles of an electrolyte necessary to cause coagulation of one litre of a colloidal solution."
As a result, a better flocculating agent will have a lower flocculating value.
Coagulation of lyophilic sols: The stability of lyophilic sols is determined by two factors. The charge and solvation of colloidal particles are these parameters.
A lyophilic sol can be coagulated when these two components are removed. This is accomplished by combining electrolytes and a suitable solvent.
Dehydration of the dispersion phase happens when solvents such as alcohol and acetone are added to hydrophilic sols. A modest amount of electrolyte can cause coagulation in this situation.
Protection of Colloids and Gold Number
Lyophilic sols are more stable than lyophobic sols.
Lyophilic colloids can prohibit any lyophobic sol from coagulating.
“The phenomenon of adding a lyophilic colloid to a lyophobic sol to keep it from coagulating is known as sol protection or colloids protection.”
Application of Colloids
Alum (coagulation) purification of water: Alum, which produces three Al ions, is added to water to coagulate the negatively charged clay particles.
Smoke Precipitation (Coagulation): Smoke is a negative sol made up of scattered carbon particles in the air.
By passing through a chamber with a highly positively charged metallic knob, these particles are eliminated.
Delta Formation (Coagulation): River water is made up of negatively charged colloidal clay particles. When a river empties into the sea, the clay particles are coagulated by the positive ions and other ions present in the water, resulting in the formation of new lands known as deltas.
Colloidal medicine: The eye creams Argyrol is colloidal silver solutions.
Photographic plates: These are thin glass plates coated with gelatin and a fine silver bromide emulsion.
Emulsion
"Colloid solutions in which both the dispersed phase and the dispersion medium are liquids are known as emulsions."
Milk, in which fat globules are spread in water, is a nice example of an emulsion. The emulsified globules are usually in the range of 10–6m in size. Emulsions have several qualities that are like lyophobic sols.
Types of Emulsion
The emulsions are classed as; depending on the nature of the dispersed phase, the emulsions are classified as;
Oil-in-water emulsions (O/W): An oil-in-water emulsion is one in which oil serves as the dispersed phase and water serves as the dispersion medium (continuous phase).
W/O emulsion (water-in-oil): A water-in-oil emulsion is an emulsion in which water serves as the dispersed phase and the oil serves as the dispersion medium. Oil emulsions are another name for these emulsions. Emulsions such as butter and cold cream are common examples.
Preparation of Emulsions
Emulsion is made by vigorously churning a mixture of the necessary oil and water, either using a high-speed mixer or ultrasonic vibrators. Simple mechanical stirring produces emulsions that are unstable.
The two constituents (oil and water) tend to separate. An appropriate stabilizing agent is usually added to achieve a stable emulsion. An emulsifier or emulsifying agent is a stabilizing chemical.
At first, the emulsifier is mixed in with the oil and water. Soaps, detergents, long-chain sulphonic acid, and lyophilic colloids such as gelatin, albumin, and casein, for example, can all operate as emulsifiers.
Applications of Emulsions
In metallurgy, ore concentration
In medicine, (Emulsion water-in-oil type)
Soap's cleansing function
Milk is a fat-in-water emulsion, which is an important part of our nutrition.
Importance of Class 12 Chemistry Surface Chemistry Notes
The Class 12 Chemistry Surface Chemistry Notes allow students to learn about the factors that affect the adsorption of gases on solids. Some of these factors are- Nature of Adsorbate, which is relatively non-specific and results in gas getting adsorbed on the surface of the solid. Then there is the Nature of Adsorbent, which Adsorbents often utilise include activated carbon, metal oxides such as silica gel, aluminium oxide, and clay. They have different adsorption properties depending on the holes. Students will learn about the pressure of gas in the form of formulas and graphs, which are easy to understand.
Students will get to know two types of catalysis namely -
Homogeneous Catalysis: This type of catalytic reaction occurs when the catalyst and the reactants are in the same phase.
Heterogeneous Catalysis: It occurs when the catalyst and the reactants are in different phases during the catalytic process.
The Surface Chemistry NEET Notes also covers characteristics of enzyme catalysis including-
They are quite effective. An enzyme molecule can convert 106 molecules of reactants every minute.
Their nature is quite specific. Urease catalysis the hydrolysis of urea alone.
They are active at optimal temperatures (298 – 310 K). The rate of an enzyme-catalysed reaction reaches to the top at a specific temperature known as the optimal temperature.
They are most active at a specific pH level known as optimal pH.
Benefits of Using Surface Chemistry NEET Notes PDF
Students using Surface Chemistry NEET Note PDF will get a complete insight about the chapter. They can learn about the three types of colloids-
Multimolecular colloids
Macromolecular colloids:
Associated colloids (Micelles)
Students can use these Surface Chemistry Class 12 Notes for the last-minute NEET preparation. Notes carry diagrams and graphs which can be really helpful to understand easily.
Every point and diagram is explained in a simple way making students understand the whole theme of the chapter. It will further help in answering questions while appearing for the NEET exam.
In case students have any doubts on any specific section of the chapter, they can refer to the Class 12 Chemistry Surface Chemistry Notes. They can even practice the chapter well.
Download Surface Chemistry Notes PDF Now
You can now download Surface Chemistry Notes PDF which is available for free on Vedantu. You just need to visit the website and download the Surface Chemistry NEET Notes PDF that you can use for the practice session.
NEET Chemistry Revision Notes - Chapter Pages
NEET Chemistry Chapter-wise Revision Notes | |
Classification of Elements and Periodicity in Properties Notes | |
Surface Chemistry Notes | |
Other Important Links Related to NEET Surface Chemistry
Other Important Links for NEET Surface Chemistry |
FAQs on Revision Notes on Surface Chemistry for NEET 2026
1. What makes Surface Chemistry Important?
Surface chemistry is vital in many critical chemical processes, including enzymatic reactions at biological interfaces found in cell walls and membranes, electronics at the surfaces and interfaces of microchips used in computers, and heterogeneous catalysts found in catalytic converters.
2. What are the methods of Preparation of Colloids?
Chemical Methods: Colloids can be created through chemical reactions that result in the creation of molecules. Sols are formed when these molecules assemble.
Bredig's Arc technique or electrical disintegration: An electric arc is struck between electrodes of the metal immersed in the dispersion solution in this process. The tremendous heat created vaporises the metal, which subsequently condenses to form colloidal particles.
Peptization: It is the process of turning a precipitate into colloidal sol by shaking it in the presence of a little amount of electrolyte with dispersion medium.
3. What are the types of surface chemistry?
There are four different types of surface chemistry. These are- Adsorption, Catalysis, Colloids, and Emulsions.
4. What are the uses of surface chemistry in daily life?
Surface chemistry is significant in everyday life because it is the foundation for many occurrences as well as technology applications.

























