
How would you convert acetylene to acetone?
Answer
478.8k+ views
Hint: To answer the following question we should know about the chemical properties and preparation of the following compound. By using them we can easily convert the following compound into the compound we need.
Complete answer:
In this question we have to convert the acetylene whose chemical formula is $ {C_2}{H_2} $ into acetone whose chemical formula is $ {C_3}{H_6}O $ . For this we first convert acetylene into $ 2 - hydroxypropanoic\;acid $ which can be formed by acetylene dilute $ {H_2}S{O_4} $ in presence of $ H{g^{2 + }} $ . The required reaction is:
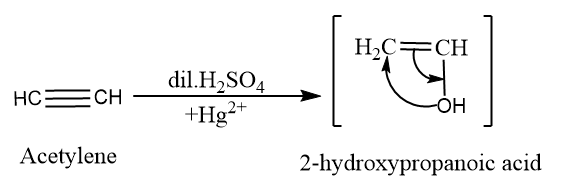
Now we will convert $ 2 - hydroxypropanoic\;acid $ into acetaldehyde by tautomerism which can be shown as:
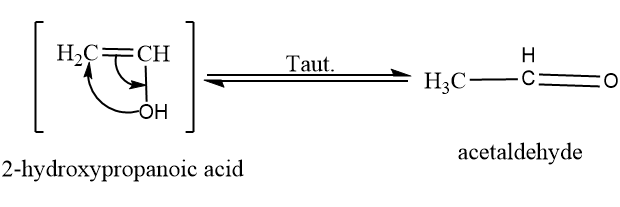
Now acetaldehyde on oxidation with acidic $ KMn{O_4} $ form acetic acid. The reaction is shown as follows:
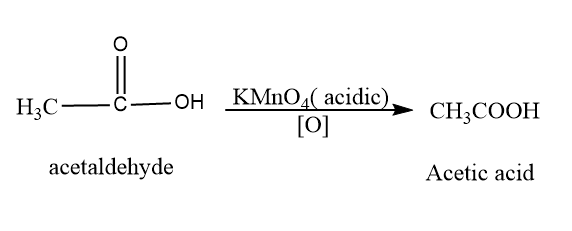
Then, we will add calcium hydroxide to acetic acid which forms calcium acetate. The reaction is:

At the last we will dry distillation the calcium acetate to form acetone, so the final product is:
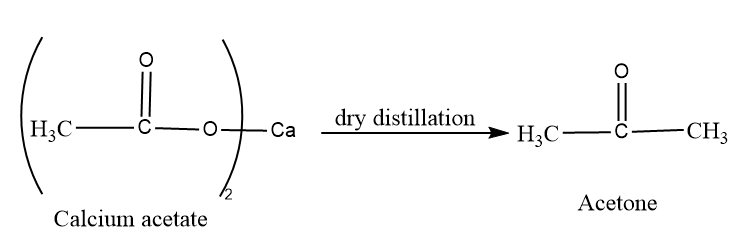
So this is the required answer.
Additional Information:
Acetones are the chemicals which can be used in the manufacture of products like nail polish remover and paint remover. Even our body makes these chemicals which break down the fat present in our body. Acetones are safe in normal amounts but can be harmful if used in excess.
Note:
Acetone is the chemical compound which has the chemical formula $ {\left( {C{H_3}} \right)_2}CO $ . It is the most simple and smallest ketone. It is colourless, flammable and a highly volatile liquid which has a pungent smell. It is used as a liquid solvent that is used to break and dissolve other substances. Some of them are used to make plastics, lacquers and textiles.
Complete answer:
In this question we have to convert the acetylene whose chemical formula is $ {C_2}{H_2} $ into acetone whose chemical formula is $ {C_3}{H_6}O $ . For this we first convert acetylene into $ 2 - hydroxypropanoic\;acid $ which can be formed by acetylene dilute $ {H_2}S{O_4} $ in presence of $ H{g^{2 + }} $ . The required reaction is:

Now we will convert $ 2 - hydroxypropanoic\;acid $ into acetaldehyde by tautomerism which can be shown as:

Now acetaldehyde on oxidation with acidic $ KMn{O_4} $ form acetic acid. The reaction is shown as follows:

Then, we will add calcium hydroxide to acetic acid which forms calcium acetate. The reaction is:

At the last we will dry distillation the calcium acetate to form acetone, so the final product is:

So this is the required answer.
Additional Information:
Acetones are the chemicals which can be used in the manufacture of products like nail polish remover and paint remover. Even our body makes these chemicals which break down the fat present in our body. Acetones are safe in normal amounts but can be harmful if used in excess.
Note:
Acetone is the chemical compound which has the chemical formula $ {\left( {C{H_3}} \right)_2}CO $ . It is the most simple and smallest ketone. It is colourless, flammable and a highly volatile liquid which has a pungent smell. It is used as a liquid solvent that is used to break and dissolve other substances. Some of them are used to make plastics, lacquers and textiles.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




