
What is the effect of temperature in angle of contact?
Answer
604.2k+ views
Hint: It is the adhesive and cohesive properties of a liquid that are responsible for the angle of contact. Define adhesion and cohesion and how it affects the contact angles and what factors change these and what is the result on the contact angle.
Complete step-by-step answer:
If there is a liquid inside a transparent vessel, and you look closely at the surface point of contact of the liquid, then you will observe the contact point of liquid with vessel is not exactly $ {{90}^{0}} $ , the angle produced will vary differently with different pairs of solid-liquid.
But why does this happen?
This is due to the phenomenon linked with the adhesive force and the cohesive force between the liquid and the material.
Adhesive force is defined as the force of attraction between molecules of two different materials. That is for water in a glass, the force between the molecules of the water and the glass at the edge is the adhesive force.
Whereas, cohesive force is the force between the molecules of the same object. That is the tendency to attract the molecules of the same material or liquid.
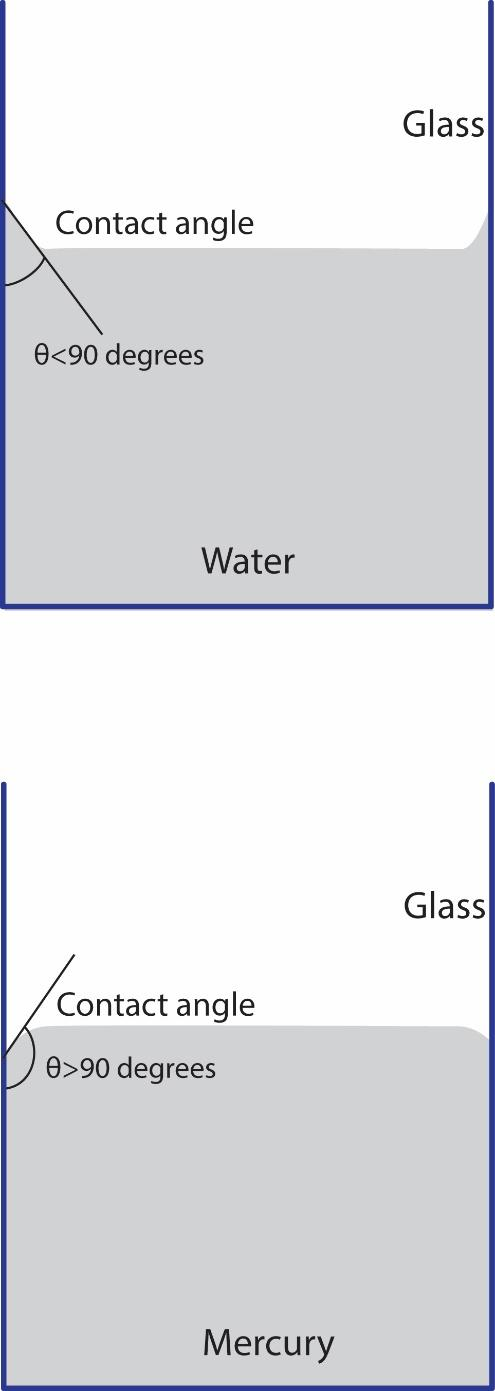
Contact angle is defined as the angle between the surface of the liquid and the outline of the contact surface.
If the adhesive force is greater than the cohesive force, the contact angle is less than $ {{90}^{0}} $ . Example of this is water and glass.
Whereas, if the adhesive force is less than the cohesive force, the contact angle is greater than $ {{90}^{0}} $ . Example of this is mercury and glass.
Temperature has an important role to play in this case.
With the rise in temperature, the cohesive forces decrease as there is more energy and vibrations of the molecules in the material. Therefore, the contact angle decreases.
That is, with the increase in temperature the contact angle decreases and with the decrease in temperature, it increases.
Note: Firstly, the contact angle between any two materials can take values from $ {{0}^{0}} $ to \[{{180}^{0}}\] degrees only. Secondly, the angle of contact is a complete surface phenomenon not a bulk property, so students should not assume that the amount of substance can change the angle of contact.
Complete step-by-step answer:
If there is a liquid inside a transparent vessel, and you look closely at the surface point of contact of the liquid, then you will observe the contact point of liquid with vessel is not exactly $ {{90}^{0}} $ , the angle produced will vary differently with different pairs of solid-liquid.
But why does this happen?
This is due to the phenomenon linked with the adhesive force and the cohesive force between the liquid and the material.
Adhesive force is defined as the force of attraction between molecules of two different materials. That is for water in a glass, the force between the molecules of the water and the glass at the edge is the adhesive force.
Whereas, cohesive force is the force between the molecules of the same object. That is the tendency to attract the molecules of the same material or liquid.
Contact angle is defined as the angle between the surface of the liquid and the outline of the contact surface.
If the adhesive force is greater than the cohesive force, the contact angle is less than $ {{90}^{0}} $ . Example of this is water and glass.
Whereas, if the adhesive force is less than the cohesive force, the contact angle is greater than $ {{90}^{0}} $ . Example of this is mercury and glass.

Temperature has an important role to play in this case.
With the rise in temperature, the cohesive forces decrease as there is more energy and vibrations of the molecules in the material. Therefore, the contact angle decreases.
That is, with the increase in temperature the contact angle decreases and with the decrease in temperature, it increases.
Note: Firstly, the contact angle between any two materials can take values from $ {{0}^{0}} $ to \[{{180}^{0}}\] degrees only. Secondly, the angle of contact is a complete surface phenomenon not a bulk property, so students should not assume that the amount of substance can change the angle of contact.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




