
In potassium, the order of energy levels is:
(A) $3s > 3d$
(B) $4s < 3d$
(C) $4s > 4p$
(D) $4s = 3d$
Answer
233.4k+ views
Hint: The electronic configuration of potassium is $[Ar]4{s^1}$. Aufbau principle states that in the ground state of atoms, the orbitals are filled in the order of their increasing energies.
Complete step by step solution:
According to the Aufbau principle, the electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only when the lower energy orbitals are filled. The order in which the energies of the orbitals increase and also the order in which the orbitals are filled are $1s,2s,2p,3s,3p,4s,3d,5s,4d,5p,6s,......$
This can be easily understood by the following diagram:
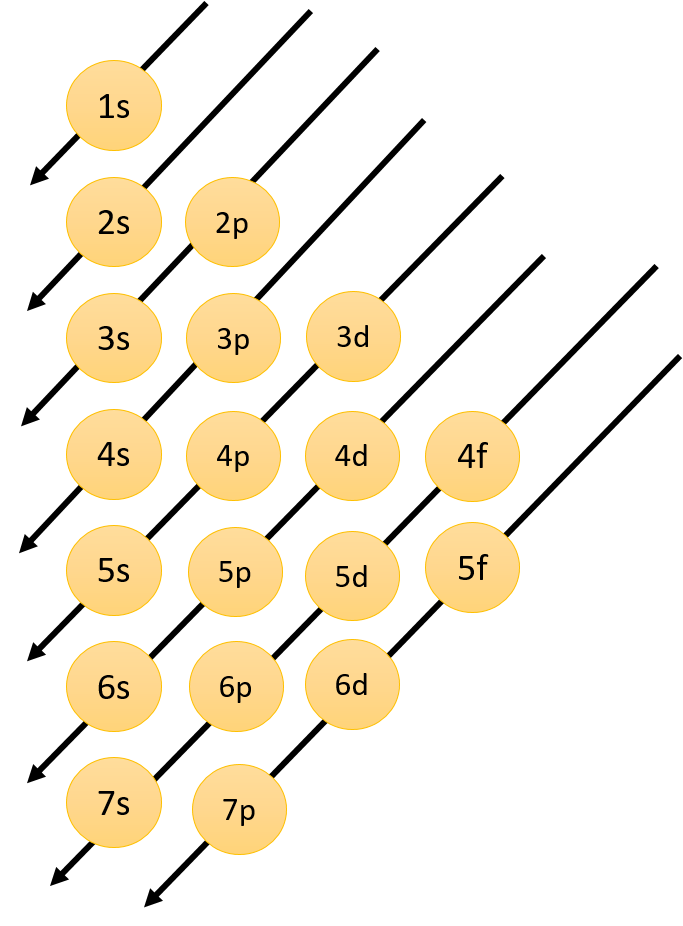
The energy of the orbitals depends upon the principal quantum number ($n$) and azimuthal quantum number ($l$). Orbital energy increases as ($n + l$) increases. So lower the value of ($n + l$) for an orbital, the lower is its energy. If there are two orbitals with the same value of ($n + l$) the one with the smaller value of $n$ has the lower energy.
Therefore a subshell with a lower value of ($n + l$) possess lower energy and is filled first. Let us consider $4s$ and $3d$ subshell.
For $4s$ subshell, $n + l = 4 + 0 = 4$,
while for 3d subshell, $n + l = 3 + 2 = 5$.
Hence, the ($n + l$) value of $4s$ subshell is lower than that of $3d$ subshell, so its energy is lower than that of $3d$ subshell. Thus, the $4s$ subshell is filled before the $3d$ subshell.
In the case of potassium ($K$) (atomic number ($Z$) =$19$), the electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}$. From the electronic configuration, it can be observed that the last electron of the potassium entered into the $4s$ subshell instead of the $3d$ subshell. This is because the energy of $4s$ subshell is lower than that of $3d$ subshell as illustrated above.
Hence, option (B) is the correct answer.
Note: The energies of different orbitals drop to a different extent with an increase in atomic number because of the effective nuclear charge. The half-filled and completely filled subshells possess extra stability due to the symmetrical distribution of electrons and greater exchange energy. That is why the electronic configuration of some elements are exceptional and not in accordance with the normal order of filling. E.g., Cr ($Z = 24$) and Cu ($Z = 29$).
Complete step by step solution:
According to the Aufbau principle, the electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only when the lower energy orbitals are filled. The order in which the energies of the orbitals increase and also the order in which the orbitals are filled are $1s,2s,2p,3s,3p,4s,3d,5s,4d,5p,6s,......$
This can be easily understood by the following diagram:

The energy of the orbitals depends upon the principal quantum number ($n$) and azimuthal quantum number ($l$). Orbital energy increases as ($n + l$) increases. So lower the value of ($n + l$) for an orbital, the lower is its energy. If there are two orbitals with the same value of ($n + l$) the one with the smaller value of $n$ has the lower energy.
Therefore a subshell with a lower value of ($n + l$) possess lower energy and is filled first. Let us consider $4s$ and $3d$ subshell.
For $4s$ subshell, $n + l = 4 + 0 = 4$,
while for 3d subshell, $n + l = 3 + 2 = 5$.
Hence, the ($n + l$) value of $4s$ subshell is lower than that of $3d$ subshell, so its energy is lower than that of $3d$ subshell. Thus, the $4s$ subshell is filled before the $3d$ subshell.
In the case of potassium ($K$) (atomic number ($Z$) =$19$), the electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}$. From the electronic configuration, it can be observed that the last electron of the potassium entered into the $4s$ subshell instead of the $3d$ subshell. This is because the energy of $4s$ subshell is lower than that of $3d$ subshell as illustrated above.
Hence, option (B) is the correct answer.
Note: The energies of different orbitals drop to a different extent with an increase in atomic number because of the effective nuclear charge. The half-filled and completely filled subshells possess extra stability due to the symmetrical distribution of electrons and greater exchange energy. That is why the electronic configuration of some elements are exceptional and not in accordance with the normal order of filling. E.g., Cr ($Z = 24$) and Cu ($Z = 29$).
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




