
The non-planar molecule among the following is:
A. \[{B_2}{H_6}\]
B. \[{C_2}{H_4}\]
C. \[{C_6}{H_6}\]
D. \[BC{l_3}\]
Answer
233.1k+ views
Hint: In order to solve this problem, we must firstly understand the Lewis structures of the compounds to identify the types of bonds and number of lone pairs present in each compound. After that, we can make the geometrical structures of the compounds, and then compare these structures.
Complete Step-by-Step answer:
To proceed with this problem, let us first draw the Lewis Structures of the given compounds:
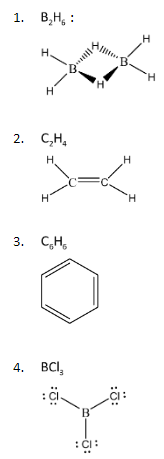
We can observe that some of these compounds are forming double bonds within the structure while some of them have lone pairs of electrons. The property of double bonds or pi – bonds that we must know is that they exist in the form of the shape of p – orbitals. P – orbitals have the shape of a two- lobe structure that expands over a given axis. Hence, these bonds have three dimensional geometries. Also, when it comes to lone pairs, these electrons always exist outside the lattice of the bond formation. Hence, existence of a lone pair, makes the over geometry of a relatively flat molecule into a 3 – dimensional structure.
From the Lewis structures represented above, the only compound with either a pi bond or any lone pairs of electrons is \[{B_2}{H_6}\].
Hence, the non – planar molecule among the given compounds is \[{B_2}{H_6}\]
Hence, Option A is the correct option.
Note: The bridging hydrogen atoms provide one electron each. The\[\;{B_2}{H_2}\] ring is held together by four electrons which form two 3-center 2-electron bonds. This type of bond is sometimes called a 'banana bond'.
Complete Step-by-Step answer:
To proceed with this problem, let us first draw the Lewis Structures of the given compounds:

We can observe that some of these compounds are forming double bonds within the structure while some of them have lone pairs of electrons. The property of double bonds or pi – bonds that we must know is that they exist in the form of the shape of p – orbitals. P – orbitals have the shape of a two- lobe structure that expands over a given axis. Hence, these bonds have three dimensional geometries. Also, when it comes to lone pairs, these electrons always exist outside the lattice of the bond formation. Hence, existence of a lone pair, makes the over geometry of a relatively flat molecule into a 3 – dimensional structure.
From the Lewis structures represented above, the only compound with either a pi bond or any lone pairs of electrons is \[{B_2}{H_6}\].
Hence, the non – planar molecule among the given compounds is \[{B_2}{H_6}\]
Hence, Option A is the correct option.
Note: The bridging hydrogen atoms provide one electron each. The\[\;{B_2}{H_2}\] ring is held together by four electrons which form two 3-center 2-electron bonds. This type of bond is sometimes called a 'banana bond'.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




