
The correct description of the Fischer projection of glyceraldehydes give below, in terms of D & L; R & S and d & l, respectively, is:
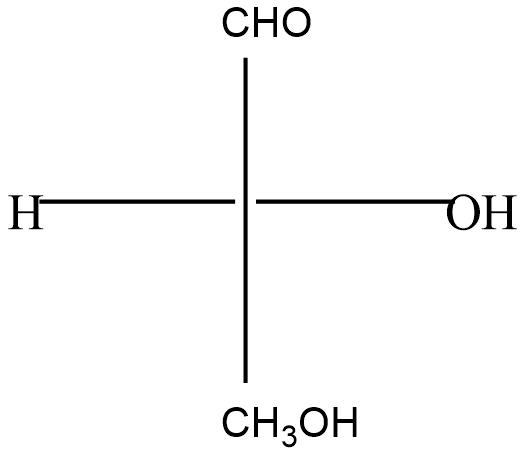
(A) D, R, d
(B) D, R, l
(C) D, S, d
(D) D, S, l

Answer
591k+ views
Hint: The right hand and left-hand nomenclature are used to name the enantiomers of a chiral compound. The stereocenters are labeled as R or S. the D-L system corresponds to the configuration of the molecule, spatial arrangement of its atoms around the chirality center. While (+) and (-) notation corresponds to the optical activity of the substance, whether it rotates the plane of polarized light clockwise (+) or counter wise (-).
Complete step by step answer:
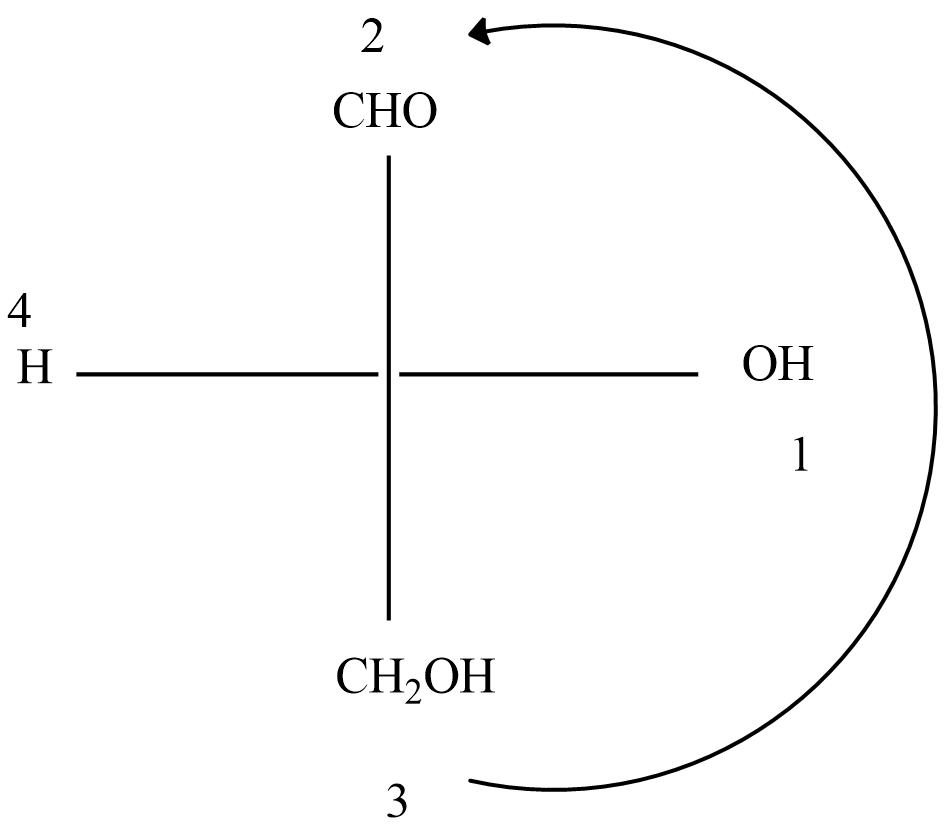
Based on R and S rotation, the above curve arrow shows a clockwise direction based on high priority substituent to low priority substituent which denotes R configuration in glyceraldehyde.
Here, priority numbering given based on the atomic number of the group. Out of four groups, the atomic number of oxygen is high in the -OH group, so it should be given first priority and marked as 1. Similarly after that group, -CHO group gives priority as 2, it goes on priority till least priority for -H atomic number. R isomer similarly equal to (d) + configuration.
Since OH is the right side of which represents D isomer.
So, the correct answer is “Option A”.
Note: A dextrorotatory compound is often prefixed with (+)- or (d)-. Likewise, a levorotatory compound is often prefixed with (-)- or (l)-. These lowercase “d-” or “l-'' prefixes are distinct from the small caps “D-” and “L-” prefixes, which are most often used to distinguish chiral organic compound and are based on the compound’s absolute configuration relative to (+)-glyceraldehyde.
Complete step by step answer:

Based on R and S rotation, the above curve arrow shows a clockwise direction based on high priority substituent to low priority substituent which denotes R configuration in glyceraldehyde.
Here, priority numbering given based on the atomic number of the group. Out of four groups, the atomic number of oxygen is high in the -OH group, so it should be given first priority and marked as 1. Similarly after that group, -CHO group gives priority as 2, it goes on priority till least priority for -H atomic number. R isomer similarly equal to (d) + configuration.
Since OH is the right side of which represents D isomer.
So, the correct answer is “Option A”.
Note: A dextrorotatory compound is often prefixed with (+)- or (d)-. Likewise, a levorotatory compound is often prefixed with (-)- or (l)-. These lowercase “d-” or “l-'' prefixes are distinct from the small caps “D-” and “L-” prefixes, which are most often used to distinguish chiral organic compound and are based on the compound’s absolute configuration relative to (+)-glyceraldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




